(UroToday.com) The 2024 ASTRO annual meeting included a session on optimizing the therapeutic ratio in prostate cancer, featuring a presentation by Dr. Abhishek Solanki discussing the initial results of F-SHARP, a multi-institutional phase 1/2 trial of focal dose-escalated salvage high dose rate (HDR) brachytherapy for radiorecurrent prostate cancer.
One-third of patients with biochemical recurrence after radiation therapy have intraprostatic radio recurrence on PSMA PET/CT, and patients with intraprostatic radio recurrence have worse metastasis-free survival, a surrogate for progression to lethal prostate cancer. Salvage local therapy for these patients allows a chance at “cure” and delay of indefinite palliative ADT, however, toxicity has historically been of concern. Salvage re-irradiation with HDR and stereotactic body radiotherapy appear to have the most favorable toxicity profile, with prior modern salvage HDR series having used 19 Gy x 1 fraction or 12-13.5 Gy x 2 fractions. Dr. Solanki and colleagues hypothesized that a dose-escalated focal salvage HDR brachytherapy approach to treating intraprostatic radio recurrence would be safe and effective.
F-SHARP is a multi-institutional phase I/II trial of focal dose-escalated salvage HDR for intraprostatic radio recurrence. Eligibility criteria included a history of localized prostate cancer treated with any form of definitive radiation therapy, and biopsy-proven intraprostatic radio recurrence with no regional or distant metastasis. All patients had a PET/CT. Brachytherapy was performed with either CT or ultrasound-based planning, and the GTV was defined using all imaging and pathologic diagnostic information. The PTV was 5-10 mm expansion on GTV (excluding organs at risk). Patients received up to 30 Gy in 1-2 fractions to a focal target, prioritizing organs and risk constraints.
The primary objective was to determine the acute radiation therapy-related grade 3+ CTCAE v4.03 genitourinary and gastrointestinal toxicity rates. Secondary endpoints included all-grade acute and late toxicity, quality of life (IPSS and EPIC-26), and biochemical/radiographic disease control and survival measures. Generalized estimating equations were used for toxicity and quality-of-life analyses. The Kaplan-Meier method was used to estimate PSA-relapse-free survival, radiographic progression-free survival, and metastasis-free survival. Cox proportional hazards models were used for univariable and multivariable analyses. From 2017-2023, there were 62 patients enrolled at three sites (Loyola University, University of Virginia, and UT Southwestern). The 2-stage phase I/II study schema is as follows: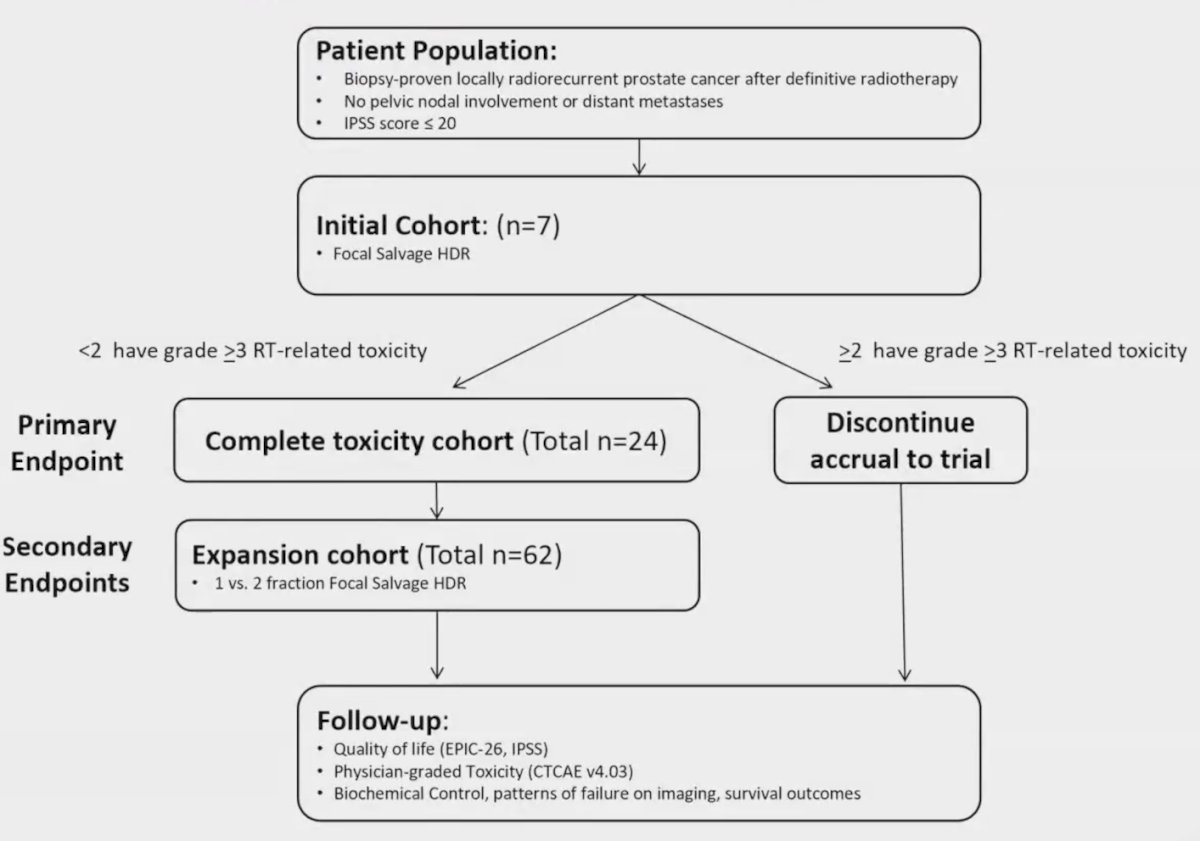
Prior radiation therapy included conventional/hypofractionated photons (60%), LDR (24%), protons (11%), and stereotactic body radiotherapy (3%). Overall, 66.1% of patients were white, and median initial PSA was 7.0 ng/mL (IQR 5.6 – 10.4):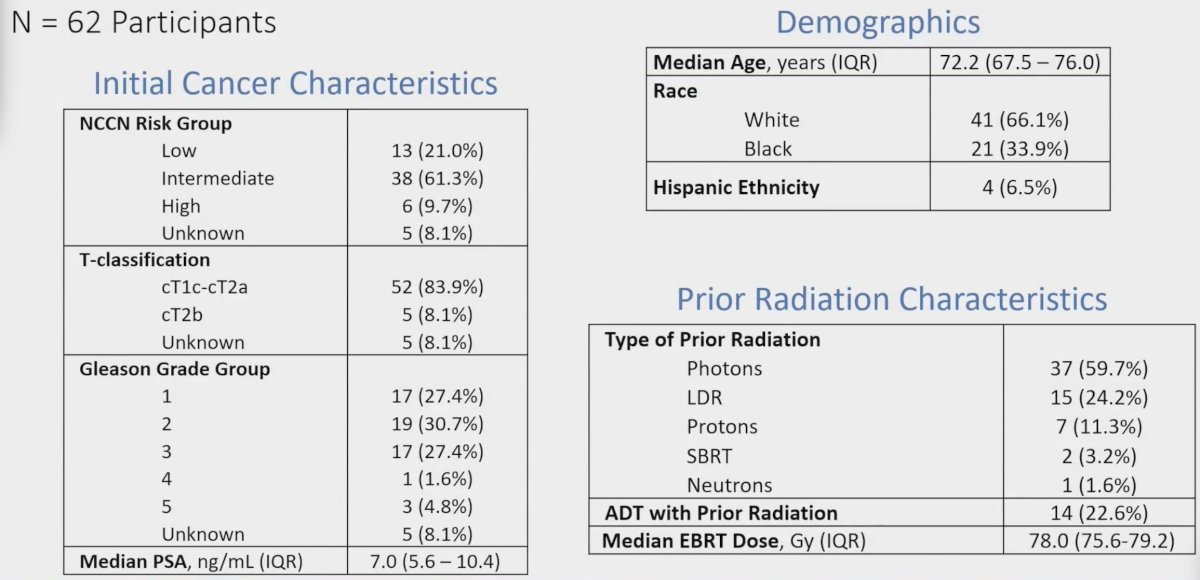
Median biochemical recurrence PSA was 4.7 ng/mL (IQR 3.7-7.3) and time from prior radiation therapy was 8 years (IQR 6.2-10.4). The median tumor D98 was 23 Gy (IQR: 20-27), for 1 fraction (n = 24) and 32 Gy (IQR: 30-34) for 2 fractions (n = 38). There were 5 patients that had concurrent hormone therapy: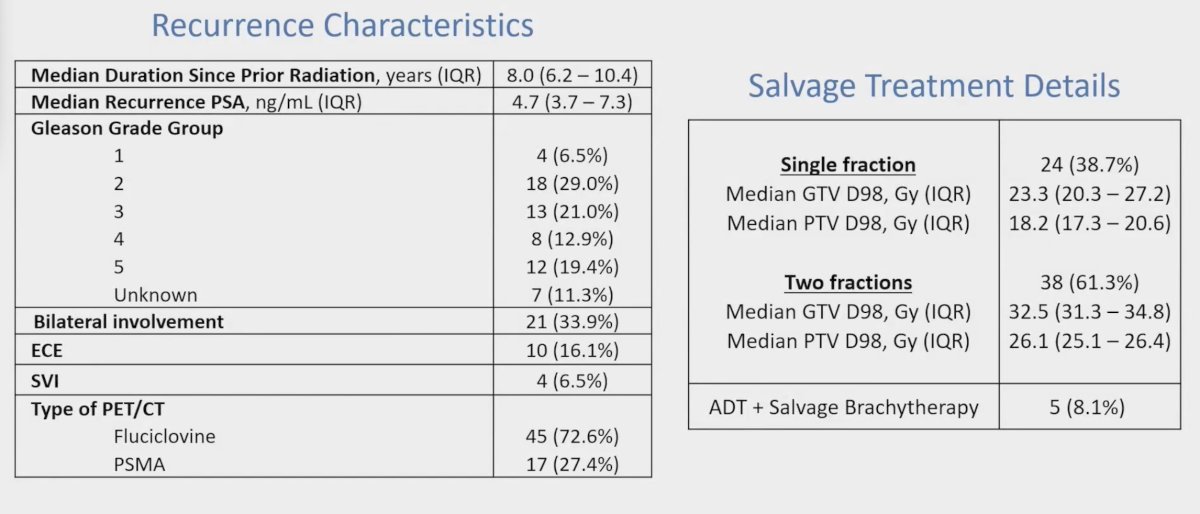
Over a median follow-up of 32.3 months, there were no grade =3 acute or late toxicities. Acute/late grade 2 genitourinary toxicity occurred in 52%/33%, acute/late grade 2 gastrointestinal toxicity occurred in 1.4%/0.3%, and acute/late grade 2 sexual toxicity occurred in 22%/24%: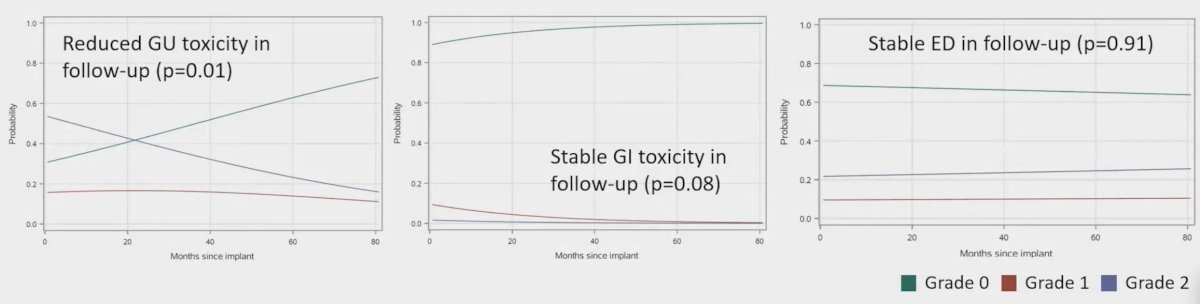
Changes in mean scores did not meet criteria for MCID in any domain: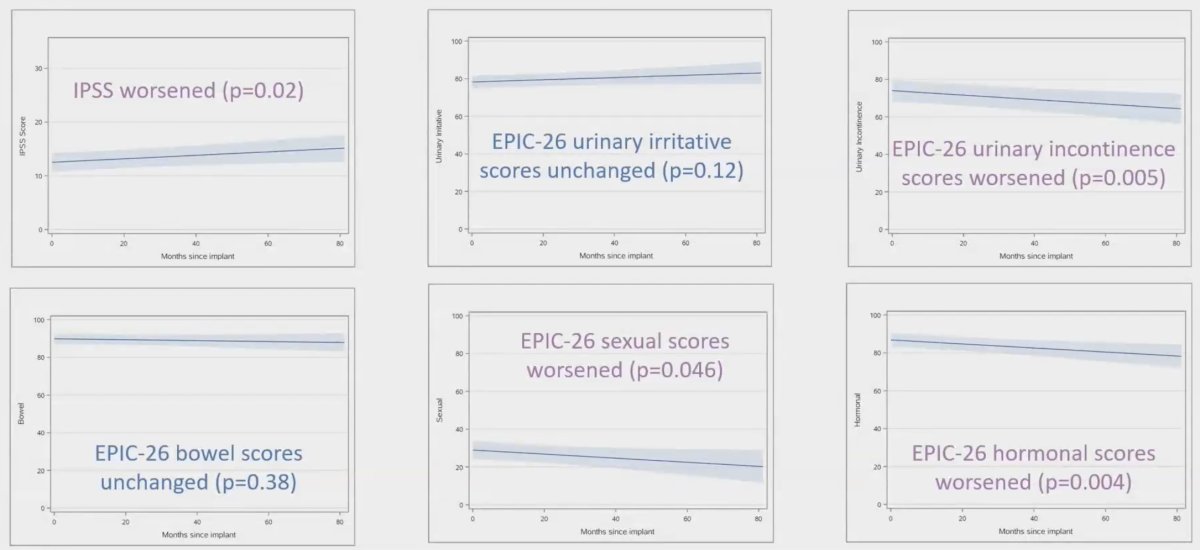
The 3-year biochemical progression-free survival was 61%, radiographic progression-free survival was 67%, locoregional recurrence-free survival was 73%, metastasis-free survival was 89%, and freedom from ADT was 93%: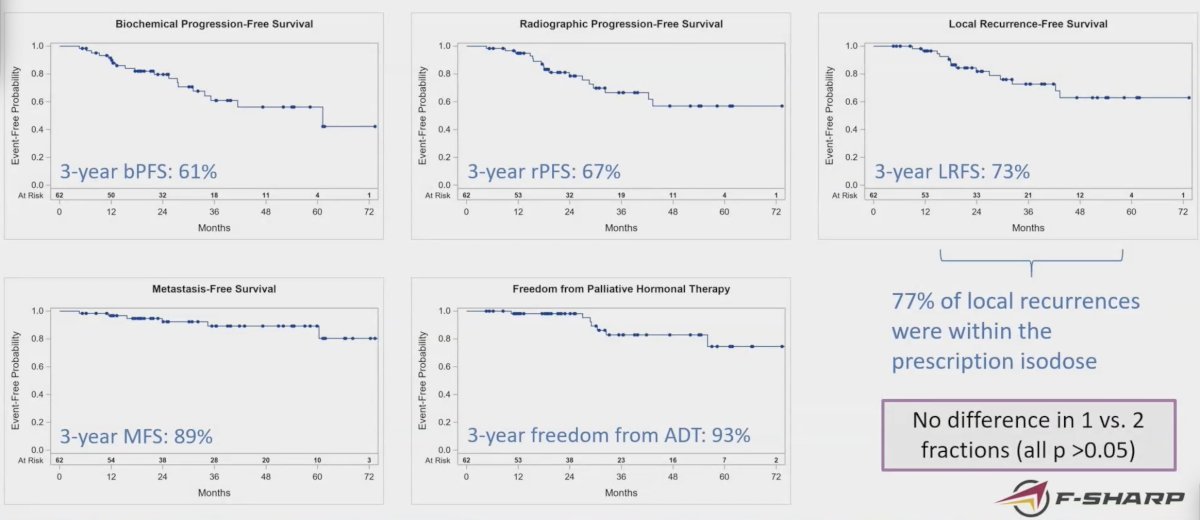
There were 5 patients that required palliative hormone therapy. There was no difference between 1 and 2 fractions for any of these events (all p > 0.05), and patients with a PSA >= 10 ng/mL were more likely to experience any event (HR 3.9, 95% CI 1.2 to 12.2; p = 0.02).
Dr. Solanki concluded his presentation discussing the initial results of F-SHARP with the following take-home points:
- Focal dose escalated salvage HDR is safe and efficacious in treating intraprostatic radio recurrence
- Correlative studies are ongoing to determine transcriptomic and clinical predictors of outcome
Presented by: Abhishek Solanki, MD, Oncologist, Loyola University Chicago Stritch School of Medicine, Maywood, IL
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting, Washington, DC, Sun, Sept 29 – Wed, Oct 2, 2024.


