Materials | Closed-Catheter Systems | Size and Length | Tips | Video Lecture | References
Overview
 Single-use sterile catheters are available for men and women with no coating and can be used with sterile lubricants.
Single-use sterile catheters are available for men and women with no coating and can be used with sterile lubricants.
Non-coated catheters are cited in the literature to cause an increase in urethral irritation, increased bacteriuria, long-term urethral complications and poor patient satisfaction.
Single-use catheters with coatings or gel are pre-coated to allow for easy insertion and removal reducing the risk of urethral mucosal irritation.
Coatings include: hydrophilic coatings, ready-to-use solution, gel on the surface, or gel in the wrapping.
Catheter Materials
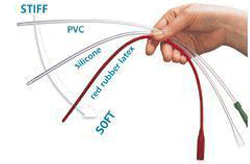 Intermittent Catheters are in two main groups: those requiring lubrication be applied before insertion and those where the coating provides the lubrication when water is applied.
Intermittent Catheters are in two main groups: those requiring lubrication be applied before insertion and those where the coating provides the lubrication when water is applied.
Catheters are packed individually in sterile packaging. As per industry standards, all disposable catheters are intended for one-time use. Uncoated catheters have frequently been re-used in the past because of cost.
Most catheters are used with a separate lubricant, although this is a matter of personal choice as some patients may just use water or nothing.
Coated catheters with hydrophilic or other coatings (such as antimicrobial or antibacterial) are sterile and not intended for reuse. Hydrophilic-coated catheters were introduced in an attempt to reduce long-term urethral complications.
The 2009 CDC guidelines have noted that coated catheters may be preferable to a standard catheter. Hydrophilic catheters are PVC catheters coated along the entire length with a hydrophilic polymer, primarily polyvinylpyrrolidone (PVP) and same with sodium chloride. When these catheters are exposed to water, the PVP coating attracts the water to the surface of the catheters, creating the biocompatible salt coating that binds the water to the surface of the catheter and forming an outer layer mainly consisting of water. This thick, slippery, smooth layer of water stays on the catheter, ensuring lubrication of the entire urethra during catheter insertion and withdrawal, thereby reducing the friction coefficient by at least 95%.
PVP is a non-allergenic material that has been used in medical devices since the 1930s.
There are also PVC-free catheters available, frequently labeled as PVC-free and DEHP-free. PVP-coated hydrophilic catheters may be indicated for patients who experience particular discomfort during catheterization when using gel-lubricated uncoated catheters or patients who have difficulty with other types of catheters. The outer layer has been designed to allow for easier insertion, minimize discomfort and reduce friction between the urethra and the catheter during intermittent catheterization. In addition, it may minimize the risk of urethral trauma and other complications.
Because of their low friction, PVP-coated catheters seem to be associated with a lesser degree of urethral inflammatory response when compared to PVC catheters. The use of hydrophilic catheters may also decrease the incidence of strictures. Nurses and patients have many hydrophilic-coated catheters from which to choose. Some available products include sterile water in the package, making it easier to activate the catheter hydrophilic coating. Some of the hydrophilic catheter utilizes water vapor inside the unique package to hydrate the catheter without adding water.
There is clinical evidence showing hydrophilic-coated catheters decrease urethral irritation and have a higher user satisfaction. In clinical practice, the reduction in the number of clinically significant UTIs is the most important issue with intermittent self-catheterization. Thus, there may be a beneficial effect regarding UTI when using hydrophilic-coated catheters. The hydrophilic-coated catheters for intermittent catheterization are intended for single use and may not be cleaned and re-used. Also the surface drying times vary by product. The design of these catheters also varies in terms of length, materials, and flexibility.
Closed-Catheter Systems
 Closed intermittent catheterization system is available and has been designed to reduce contamination of the bladder because the catheter never comes in direct contact with the inserter’s hands.
Closed intermittent catheterization system is available and has been designed to reduce contamination of the bladder because the catheter never comes in direct contact with the inserter’s hands.
These systems are designed for single use only.
These systems include pre-lubricated products with an integrated (all-in-one) collection bag, give flexibility for the user and are efficient for hospital use. Some closed systems are packaged as a sterile kit containing all the equipment required to do aseptic catheterization (for example, when traveling or while at work)
Most systems have an introducer tip that is passed through a pre-lubricated plastic sleeve or guide, keeping the catheter straight and lubricated as it is advanced. When the plastic sleeve is squeezed, it prevents the catheter from slipping during insertion.
The 15 mm introducer tip on the closed system has been shown to bypass the distal urethra where harmful bacteria reside which is key in the prevention of UTI.
Catheter Size and Length
 Catheter sizes available for intermittent catheterization are similar to those available for an indwelling urinary catheter.
Catheter sizes available for intermittent catheterization are similar to those available for an indwelling urinary catheter.
Catheter diameter is measured in French (Fr or Ch) units, and sizes range from 6 to 12 Fr for children and 14 to 22 Fr for adults.
The funnel end of the catheter is usually color-coded to easily identify Fr size. Intermittent catheters have different lengths and are gender-specific.
Catheters with lengths of approximately 12 inches (about 40 cm) allow for adequate passage through a male urethra.
Women and children, whose urethras are shorter in length, may find a shorter-length catheter of 6 to 12 inches (20 to 40 cm), which is easier to grasp and manipulate because it will not loop or kink, thus allowing easy flow and urine drainage through the catheter.
Catheter Tips
 The tip of a catheter used for intermittent catheterization can be either straight or curved (referred to as Coudé-tipped or Tiemann).
The tip of a catheter used for intermittent catheterization can be either straight or curved (referred to as Coudé-tipped or Tiemann).
Most straight tipped catheters are tapered so they can pass smoothly through the urethra.
Using a curved catheter tip in the male patient with an enlarged prostate or a narrowed urethra (for example, from a stricture) may allow for ease of insertion.
In the United States, some clinicians advocate the use of an introducer tip when performing intermittent self-catheterization. The introducer tip was first studied in the 1990s. These tips, found in closed-catheter systems, were originally used in obstetric patients, but have been tested in acute care and rehabilitation hospitals.
By inserting this tip in the urethra before advancing the catheter, the first portion (1.5 cm) of the distal urethra is bypassed. This portion of the distal urethra can be colonized with perineal bacteria, particularly E. coli. Colonization of pseudo monas and klebsiella frequently occurs in the perineum and urethra in men with a spinal cord injury.
To bypass the distal urethra area, the catheter is advanced into the introducer tip, the tip is inserted into the distal urethra, and then the catheter is passed through the tip into the urethra. This prevents contamination of the catheter and introduction of bacteria into the bladder.
There is evidence that the use of an introducer-tipped catheter reduced UTI in hospitalized patients with spinal cord injury on intermittent catheterization.
References
Bennett, C.J., Young, M.N., et al. The effect of urethral introducer tip catheters on the incidence of urinary tract infection outcomes in spinal cord injured patients, Journal of Urology, 1997;158(2),p 519-521.
Charbonneau-Smith, R. No-touch catheterization and infection rates in a select spinal cord injured population. Rehabilitation Nursing, 1993;18(5), 296-299.
Fader, M., Moore, K.N., et al. Coated catheters for intermittent catheterization: Smooth or sticky? British Journal of Urology International, 2001; 88, p 373-377.
Getliffe, K., Fader, M., et al. Current evidence on intermittent catheterization: Sterile single-use catheters or clean reused catheters and the incidence of UTI. Journal of Wound Ostomy Continence Nursing, 2007; 34(3), 289-296.
Giannantoni, A., DiStasi, S., et al. Intermittent catheterization with a prelubricated catheter in spinal cord injured patients: A prospective randomized crossover study. Journal of Urology, 2002; 166, p 130-133.
Gray, M. Are all urethral catheters created equal? Journal of Urology,2009; 182, p 2561-2562.
Gould, C.V., Umscheid, C.A., Agarwal, R.K., Kuntz, G., Pegues, D.A., and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for the prevention of catheter-associated urinary tract infections 2009. Retrieved from http://www.cdc.gov/ncidod/ dhqp/pdf/guidelines/CAUTI_Guideli ne2009final.pdf
Hedlund, H., Hjelmås, K., Jonsson, O., Klarskov, P., & Talja, M. Hydrophilic versus non-coated catheters for intermittent catheterization. Scandinavian Journal of Urology and Nephrology, 2001; 35, p 49–53.
Moore KN, Burt J, Voaklander DC. Intermittent catheterization in the rehabilitation setting: a comparison of clean and sterile technique. Clin Rehabil 2006;20:461-8.
Moore KN, Fader M, Getliffe K. Long-term bladder management by intermittent catheterisation in adults and children. Cochrane Database Syst Rev 2007;(4):CD006008.
National Institute for Health and Clinical Excellence. The guidelines manual 2009. www. nice.org.uk/aboutnice/howwework/developingniceclinicalguidelines/ clinicalguidelinedevelopmentmethods/GuidelinesManual2009.jsp.
Newman, D.K., & Wein, A.J. Managing and treating urinary incontinence (2nd ed). 2009 Baltimore: Health Professions Press.


