(UroToday.com) The 2023 American Urological Association (AUA) annual meeting held in Chicago, IL between April 28 and May 1st, 2023, was host to a non-invasive bladder cancer podium session. Dr. Roger Li presented the results of CORE1, a phase 2 single arm study of CG0070 combined with pembrolizumab in patients with non-muscle invasive bladder cancer unresponsive to Bacillus Calmette-Guerin (BCG).
Cretostimogene Grenadenorepvec (CG0070) is a cancer-selective oncolytic adenovirus engineered to preferentially replicate in tumor cells. The E2F Promoter along with GM-CSF transgene are inserted into wild-type adenovirus backbone, with the function of each gene/protein summarized in the table below: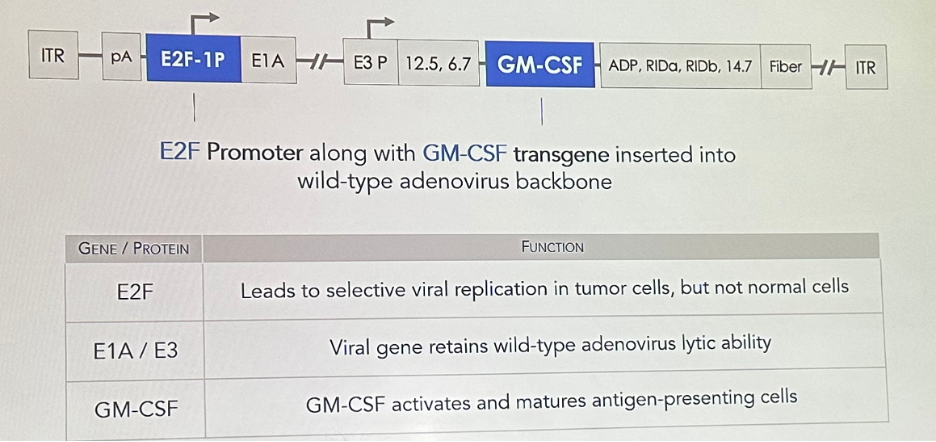
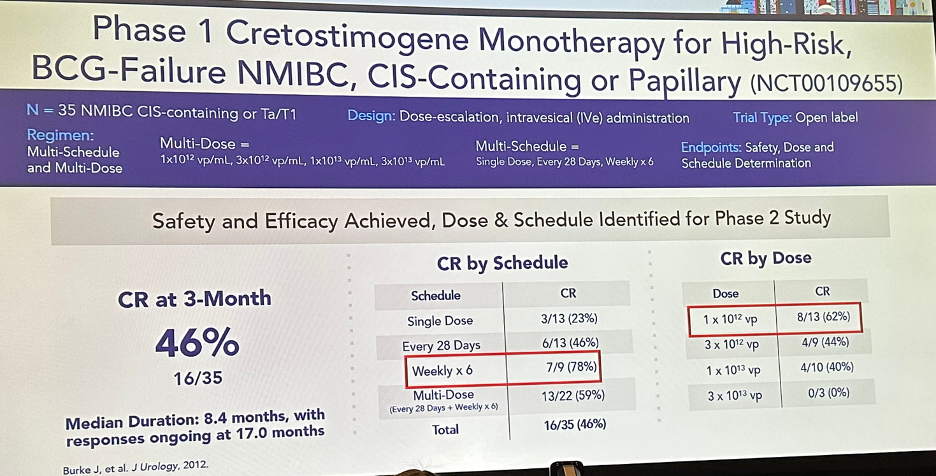
CG0070 monotherapy has previously been evaluated in a phase 1 trial (V0046) of 35 patients with high-risk, BCG-failure NMIBC (CIS-containing or papillary). CG0070 demonstrated a complete response rate of 46% at 3 months and met the safety/efficacy outcomes, with the target dose and schedule identified for a subsequent phase 2 study.
Next, CG0070 monotherapy was evaluated in an open label, phase 2 trial (BOND-002) of 46 patients with BCG-failure NMIBC (CIS containing). A CR at any time was achieved in 30/46 patients (65%), with durable CRs of 44% and 28% at 6 and 12 months, respectively.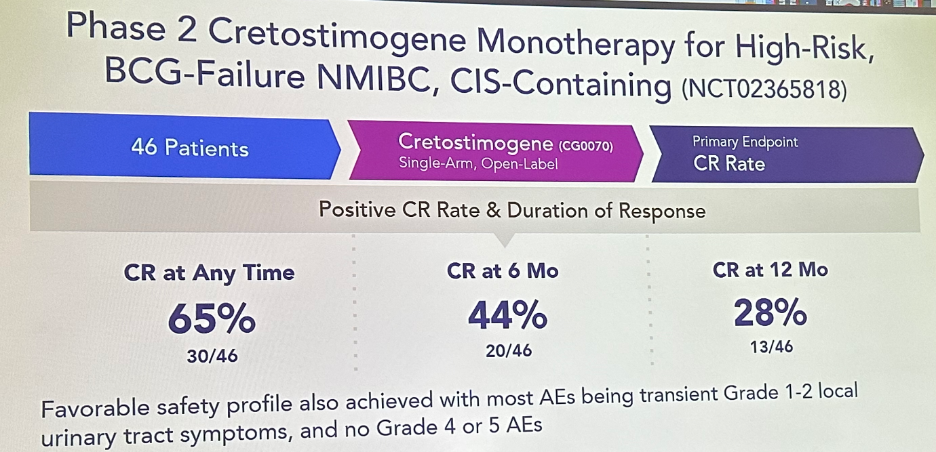
Based on these promising results, the phase 3 BOND-003 trial will evaluate CG0070 monotherapy for patients with high-risk, BCG-unresponsive NMIBC (CIS-containing).
Given the mechanism of action of CG0070, as illustrated in the figure below, it has been hypothesized that CG0070 and pembrolizumab (PD-1 inhibitor) may have synergistic mechanisms of action.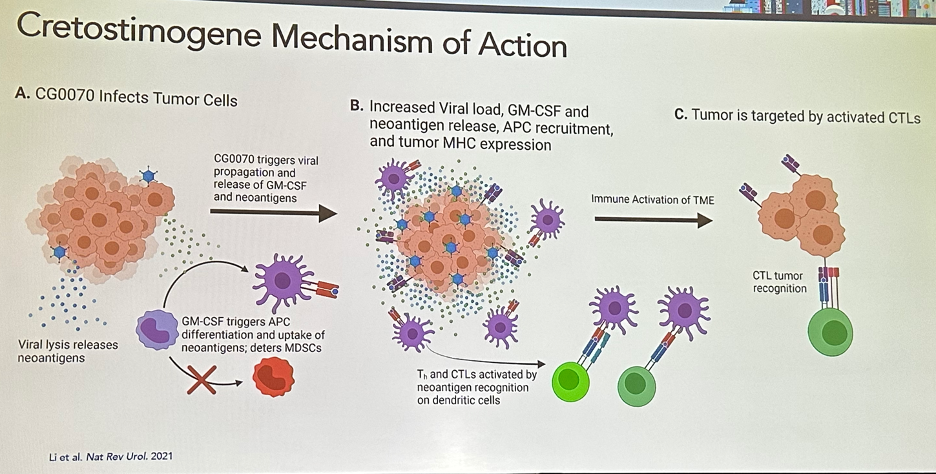
As such, CORE1 was designed as an open label, single arm Phase 2 trial evaluating the combination of CG0070 plus pembrolizumab in 35 patients with high-risk, BCG-unresponsive NMIBC (CIS-containing). All patients received CG0070 induction weekly (1x1012 vp/mL) for 6 weeks, followed by a second induction course weekly for 3 weeks in responders and 6 weeks in non-responders. All responders subsequently received a maintenance course weekly for 3 weeks. Patients concurrently received pembrolizumab every 6 weeks (as opposed to the usual 3 weeks) at a dose of 400 mg through year 2. The study endpoints were CR at any time and 12 months (duration of response).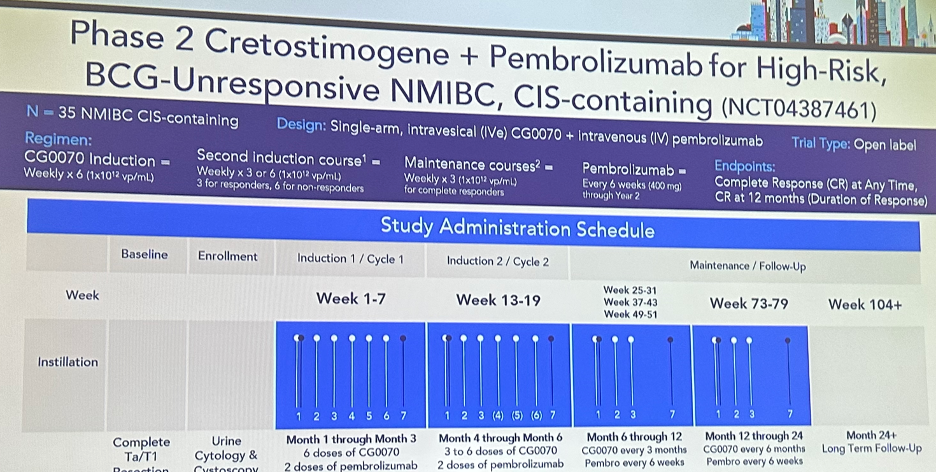
Baseline patient characteristics are summarized below. The median number of prior BCG instillations was 12 (range: 9 – 30). 60% had persistent high-risk NMIBC, with the remaining 40% having recurrent high-risk NMIBC. Of note, 80% of patients had pure CIS.
The swimmer’s plot for the study subjects is demonstrated below. Impressively, an overall CR rate of 85% was observed. This was longitudinally maintained with 6-, 9-, and 12-month CR rates of 82%, 81%, and 68%, respectively.
With regards to adverse events:
- Predominantly transient, grade 1-2 local GU AEs
- Grade 3 AEs were observed in 4 patients (11%)
- No grade 4-5 AEs were observed
- AE profile was generally consistent with prior studies of each agent alone
- No clear evidence of additive/synergistic toxicity
Dr. Li concluded as follows:
- Monotherapy activity of CG0070 in NMIBC after BCG failure has been established in the past 2 studies of CG0070: V0046 and BOND-002
- The combination of CG0070 and pembrolizumab appears to be highly active in BCG-unresponsive NMIBC based on preliminary results of the CORE-001 study
- Studies of CG alone (Phase 3 BOND-003, ongoing study) and in combination with checkpoint inhibitor therapy (Phase 3 PIVOT-001, planned study) will further elucidate the potential role of CG0070-based therapy for BCG-unresponsive NMIBC
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Urological Association (AUA) Annual Meeting, Chicago, IL, April 27 – May 1, 2023
- Burke J, et al. A first in human phase 1 study of CG0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol, 2012.
- Packiam, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol, 2018.
Gene Therapy and Pembrolizumab in BCG-Unresponsive CIS-Containing Population: Updated Results, Efficacy, and Correlative Studies - Roger Li


