Bladder Cancer LR
Bladder Cancer LR
Promoting BCAN’s 2025 Walks to End Bladder Cancer: Join the Fight Against Bladder Cancer
Highlights from ESMO’24 Presidential Symposium: Bladder Cancer in the Forefront
Systemic Treatment in Bladder Cancer State of Art in 2024 and Challenges in Morocco
Bladder cancer treatment has evolved from traditional surgery and chemotherapy to include immunotherapy, targeted therapies, and antibody drug conjugates. These therapeutic innovations, along with advances in surgical techniques and multimodal approaches, continue to reshape clinical practice and improve outcomes for bladder cancer patients. However, the high cost of these treatments poses a significant challenge in low-income countries such as Morocco.
IBCG Retreat 2024
American Society of Clinical Oncology (ASCO) 2024 Bladder Cancer Updates
EV+P nearly doubled median PFS and OS versus platinum-based chemotherapy in patients with previously untreated locally advanced or metastatic urothelial cancer in the phase 3 EV-302 trial and is NCCN category 1 and ESMO guidelines preferred treatment option. PRO assessments included the
Racial Disparities in Bladder Cancer
Gem/Doce in 2024: What is Next?
- Written by: Vignesh Packiam, MD, Associate Professor, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ
International Bladder Cancer Group (IBCG) at the American Urological Association (AUA) 2024
- Written by: Bogdana Schmidt, MD, MPH, Assistant Professor Urologic Oncology, University of Utah, Huntsman Cancer Institute, Salt Lake City, Utah
European Association of Urology (EAU) 2024: Bladder Cancer Highlights
- Written by: Amanda Myers, MD, Fellow of Urologic Oncology, MD Anderson Cancer Center, Houston, Texas
American Urological Association (AUA) 2024 Annual Meeting Summary
- Written by: Patrick J. Hensley, MD, Urologic Oncologist, University of Kentucky College of Medicine Lexington, KY, USA
Cretostimogene Grenadenorepvec: At the CORE and Forming BONDs in High-Risk NMIBC and PIVOTing into Intermediate Risk NMIBC
Bladder cancer remains the sixth most commonly diagnosed cancer in the United States, with an estimated 82,290 incident cases in 2023.1 Because of the persistent recurrence risk of NMIBC in a highly comorbid population, there has been an FDA-led drive towards developing novel treatment options for these patients. The following article will highlight recent advances in this disease space with a specific focus on the oncolytic adenovirus agent cretostimogene grenadenorepvec, and the registration trial in intermediate risk non-muscle invasive bladder cancer (NMIBC), PIVOT-006.
- Written by: Zachary Klaassen, MD, MSc, Wellstar MCG Health Georgia Cancer Center Augusta, Georgia, USA
- References:
- American Cancer Society. Key Statistics for Bladder Cancer. https://www.cancer.org/cancer/types/bladder-cancer/about/key-statistics.html#:~:text=time%20of%20diagnosis-,How%20common%20is%20bladder%20cancer%3F,men%20and%204%2C550%20in%20women. Accessed on December 1, 2023.
- Burke JM, Lamm DL, Meng MV, et al. A first in human phase 1 study of GC0070, a GM-CSF expressing oncolytic adenovirus, for the treatment of nonmuscle invasive bladder cancer. J Urol. 2012 Dec;188;(6):2391-2397.
- Packiam VT, Lamm DL, Barocas DA, et al. An open label, single-arm, phase II multicenter study of the safety and efficacy of CG0070 oncolytic vector regimen in patients with BCG-unresponsive non-muscle-invasive bladder cancer: Interim results. Urol Oncol. 2018 Oct;36(10):440-447.
Emerging Therapeutic Options for Low-Grade Non-Invasive Bladder Cancer: Primary Chemoablation
Introduction
Bladder cancer remains the sixth most commonly diagnosed cancer in the United States, with an estimate of 82,290 incident cases in 2023.1 At diagnosis, approximately 75% of patients present with non-muscle invasive disease, with significant clinical heterogeneity observed within this disease group.2,3 Patients with initial low-grade Ta disease (i.e., confined to the mucosal lining) represent a unique patient cohort given their favorable long-term oncologic outcomes, given that they are more likely to recur than progress to life-threatening disease.4
- Written by: Rashid K. Sayyid, MD, MSc University of Toronto Toronto, ON and Zachary Klaassen, MD, MSc Medical College of Georgia Augusta, Georgia, USA
- References:
- American Cancer Society. Key Statistics for Bladder Cancer.
- Babjuk M, Burger M, Comperat EM, et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 update. Eur Urol 2019; 76(5):639-57.
- Monteiro LL, Witjes JA, Agarwal PK, et al. ICUD-SIU International Consultation on Bladder Cancer 2017: management of non-muscle invasive bladder cancer. World J Urol 2019; 37(1):51-60.
- Hernandez V, Llorente C, de la Pena E, et al. Long-term oncological outcomes of an active surveillance program in recurrent low grade Ta bladder cancer. Urol Oncol 2016; 34(4):165.e19-23.
- Mossanen M, Gore JL. The Burden of Bladder Cancer Care – Direct and Indirect Costs. Curr Opin Urol 2014; 24(5):487-91.
- Zhou Z, Zhao S, Lu Y, et al. Meta-analysis of efficacy and safety of continuous saline bladder irrigation compared with intravesical chemotherapy after transurethral resection of bladder tumors. World J Urol, 2019; 37(6):1075.
- Sylvester RJ, et al. Systematic Review and Individual Patient Data Meta-analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa-pT1 Urothelial Carcinoma of the Bladder: Which Patients Benefit from the Instillation? Eur Urol 2016; 69(2): 231-44.
- Perlis N, Zlotta AR, Beyene J, et al. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: an updated meta-analysis on 2548 patients and quality-of-evidence review. Eur Urol 2013; 64(3):421-30.
- Messing EM, Tangen CM, Lerner SP, et al. Effect of Intravesical Instillation of Gemcitabine vs Saline Immediately Following Resection of Suspected Low-Grade Non-Muscle-Invasive Bladder Cancer on Tumor Recurrence: SWOG S0337 Randomized Clinical Trial. JAMA 2018; 319(18):1880-8.
- Arends TJH, Nativ O, Maffezzini M, et al. Results of a Randomised Controlled Trial Comparing Intravesical Chemohyperthermia with Mitomycin C Versus Bacillus Calmette-Guérin for Adjuvant Treatment of Patients with Intermediate- and High-risk Non-Muscle-invasive Bladder Cancer. Eur Urol 2016; 69(6):1046-52.
- Arends TJH, van der Heijdem AG, Witjes JA. Combined chemohyperthermia: 10-year single center experience in 160 patients with nonmuscle invasive bladder cancer. J Urol 2014; 192(3):708-13.
- Shelley MD, Kynaston H, Court J, et al. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int 2001; 88(3):209-16.
- Han RF, Pan JG. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology 2006; 67(6):1216-23.
- Shelley MD, Wilt TJ, Court J, et al. Intravesical bacillus Calmette-Guérin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. BJU Int 2004; 93(4):485-90.
- Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol 2003; 169(1):90-5.
- Popert RJ, Goodall J, Coptcoat MJ, et al. Superficial bladder cancer: the response of a marker tumour to a single intravesical instillation of epirubicin. Br J Urol 1994; 74(2):195-9.
- Mostafid AH, Porta N, Cresswell J, et al. CALIBER: a phase II randomized feasibility trial of chemoablation with mitomycin‐C vs surgical management in low‐risk non‐muscle‐invasive bladder cancer. BJU Int 2020; 125(6):817-26.
- Lindgren MS, Bue P, Azawi N, et al. The DaBlaCa-13 Study: Short-term, Intensive Chemoresection Versus Standard Adjuvant Intravesical Instillations in Non-muscle-invasive Bladder Cancer-A Randomised Controlled Trial. Eur Urol 2020; 78(6):856-62.
- Kleinmann N, Matin SF, Pierorazio PM, et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial. Lancet Oncol 2020; 21(6):776-85.
- FDA approves mitomycin for low-grade upper tract urothelial cancer.
- Chevli KK, Shore ND, Trainer A, et al. Primary Chemoablation of Low-Grade Intermediate-Risk Nonmuscle-Invasive Bladder Cancer Using UGN-102, a Mitomycin-Containing Reverse Thermal Gel (Optima II): A Phase 2b, Open-Label, Single-Arm Trial. J Urol 2022; 207(1):61-9.
- Prasad SM, Huang WC, Shore ND, et al. Treatment of Low-grade Intermediate-risk Nonmuscle-invasive Bladder Cancer With UGN-102 ± Transurethral Resection of Bladder Tumor Compared to Transurethral Resection of Bladder Tumor Monotherapy: A Randomized, Controlled, Phase 3 Trial (ATLAS). J Urol 2023; 101097JU0000000000003645.
BCG-unresponsive Non-Muscle Invasive Bladder Cancer: Immune Checkpoint Inhibitors
Introduction
Intravesical Bacillus Calmette-Guerin (BCG) currently remains the standard-of-care, guideline recommended treatment of choice in the adjuvant setting for intermediate- and high-risk non-muscle invasive bladder cancer (NMIBC) due to its ability to reduce the risk of disease recurrence and, more importantly, disease progression.1-3 However, despite adequate BCG treatment, defined as receipt of at least five doses of the initial six-dose induction course and at least 2/3 maintenance doses or at least 2/6 doses of the second induction course, up to 50% of such patients will develop a BCG-refractory, relapsing, or failure state.4 Currently, radical cystectomy remains the gold standard approach in this setting.1 However, many patients are either unfit or refuse cystectomy. As such, bladder-sparing approaches in this setting are of utmost importance.
- Written by: Rashid K. Sayyid, MD, MSc University of Toronto Toronto, ON and Zachary Klaassen, MD, MSc Medical College of Georgia Augusta, Georgia, USA
- References:
- EAU Guidelines: Non-muscle-invasive Bladder Cancer.
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766-73.
- Schmidt S, Kunath F, Coles B, et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Review. 2020;1(1):CD011935.
- Babjuk M, Burger M, Comperat EM, et al. European Association of Urology Guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) - 2019 Update. Eur Urol. 2019;76(5):639–57.
- Steinberg R, Bahnson R, Brosman S, et al. Efficacy and Safety of Valrubicin for the Treatment of Bacillus Calmette-Guerin Refractory Carcinoma in Situ of the Bladder. J Urol. 200;163(3):761-7.
- Bacillus Calmette-Guérin-unresponsive nonmuscle invasive bladder cancer: developing drugs and biologics for treatment guidance for industry. 2018.
- FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer.
- FDA D.I.S.C.O. Burst Edition: FDA approval of Adstiladrin (nadofaragene firadenovec-vncg) for patients with high-risk Bacillus Calmette-Guérin unresponsive non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumors.
- Kates M, Matoso A, Choi W, et al. Adaptive Immune Resistance to Intravesical BCG in Non–Muscle Invasive Bladder Cancer: Implications for Prospective BCG-Unresponsive Trials. Clin Cancer Res. 2020;26(4):882-91.
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22(7):919-30.
- Necchi A, Roumiguié M, Esen AA, et al. Pembrolizumab (pembro) monotherapy for patients (pts) with high-risk non–muscle-invasive bladder cancer (HR NMIBC) unresponsive to bacillus Calmette–Guérin (BCG): Results from cohort B of the phase 2 KEYNOTE-057 trial. J Clin Oncol. 2023;41(Supp 6):LBA442.
- Alanee S, Sana S, El-Zawahry A, et al. Phase I trial of intravesical Bacillus Calmette-Guérin combined with intravenous pembrolizumab in recurrent or persistent high-grade non-muscle-invasive bladder cancer after previous Bacillus Calmette-Guérin treatment. World J Urol. 2021;39(10):3807-13.
- Meghani K, Cooley LF, Choy B, et al. First-in-human Intravesical Delivery of Pembrolizumab Identifies Immune Activation in Bladder Cancer Unresponsive to Bacillus Calmette-Guérin. Eur Urol. 2022;82(6):602-10.
- Li R, Sexton WJ, Dhillon J, et al. A Phase 2 Study of Durvalumab for Bacillus Calmette-Guerin (BCG) Unresponsive Urothelial Carcinoma In Situ of the Bladder. Clin Cancer Res. 2023.
- Kowalski M, Guindon J, Brazas L, et al. A phase II study of oportuzumab monatox: an immunotoxin therapy for patients with noninvasive urothelial carcinoma in situ previously treated with bacillus Calmette-Guérin. J Urol. 2012;188(5):1712-8.
- Gurram S, Bellfield S, Dolan R, et al. PD09-04 Interim analysis of a phase I single-arm study of the combination of durvalumab (Medi4736) and vicinium (Oportuzumab Monatox, Vb4-845) in subjects with high-grade non-muscle-invasive bladder cancer previously treated with Bacillus Calmette-Guerin (Bcg). J Urol. 2021;206(Suppl 3):e120.
- Black PC, Tangen C, Singh P, et al. Phase II trial of atezolizumab in BCG-unresponsive non-muscle invasive bladder cancer: SWOG S1605 (NCT #02844816). J Clin Oncol;2020(Suppl):5022.
- Shore ND, Powles T, Bedke J, et al. A phase 3 study of the subcutaneous programmed cell death protein 1 inhibitor sasanlimab as single agent for patients with bacillus Calmette-Guérin, unresponsive high-risk, non-muscle invasive bladder cancer: CREST Study Cohort B. J Clin Oncol. 2022;40(Suppl 16):40.
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2021;384:2102-14.
- Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109(8):1499-505.
- Hudolin T, Mengus C, Coulot J, et al. Expression of Indoleamine 2,3-Dioxygenase Gene is a feature of poorly differentiated non-muscle-invasive urothelial cell bladder carcinomas. Anticancer Res. 2017;37(3):1375-80.
- Tabernero, et al. BMS-986205, an indoleamine 2,3-dioxygenase 1 inhibitor (IDO1i), in combination with nivolumab (NIVO): Updated safety across all tumor cohorts and efficacy in pts with advanced bladder cancer (advBC). J Clin Oncol. 2018;36(Suppl 15):4512.
BCG-Unresponsive Non-Muscle Invasive Bladder Cancer: Review of Intravesical Chemotherapy and Photodynamic Therapy
Introduction
Intravesical Bacillus Calmette-Guerin (BCG) remains the current standard-of-care, guideline-recommended treatment of choice in the adjuvant setting for intermediate- and high-risk non-muscle invasive bladder cancer (NMIBC) due to its ability to reduce the risk of disease recurrence and, more importantly, disease progression.1-3 Despite adequate BCG treatment up to 50% of patients will develop a BCG-refractory, relapsing, or failure state.4 Over the last several years, there has been a plethora of data in the BCG unresponsive disease space, leading to FDA approvals for pembrolizumab in January 2020 and nadofaragene firadenovec in December 2022.6 Many of these novel immune-based treatments overcome some of the limitations of older agents in this setting,7 particularly those related to short durations of exposure limiting their efficacy.8 This same concept of extending the intravesical exposure time has recently been extrapolated to the intravesical chemotherapeutic treatment landscape, where we have witnessed the emergence of novel agents with prolonged mechanisms of action and alternate methods of administration. In this Center of Excellence article, we will discuss the currently available evidence and ongoing studies for intravesical chemotherapeutic agents and photodynamic therapy in the BCG unresponsive NMIBC disease space.
Gemcitabine + Docetaxel
In 2020, the results of a multicenter, retrospective analysis evaluating sequential gemcitabine plus docetaxel in patients with recurrent NMIBC and a history of intravesical BCG treatment were reported. In this study, all patients received intravesical gemcitabine (1 gm/50 ml sterile water or saline) for 60 to 90 minutes, after which it was drained and intravesical docetaxel (37/5 mg/50 ml saline) was instilled and held for 1-2 hours. To minimize side effects secondary to the irritative effects of gemcitabine (pH 2.5), 1,300 mg of oral sodium bicarbonate was given the evening before and the morning of each instillation to alkalinize the urine. The induction regimen was given once weekly for 6 weeks, followed by monthly maintenance for up to 24 months in the majority of the participating institutions. Surveillance was performed in accordance with institutional/national guidelines.This analysis included 276 patients with a median age of 73 years. The median number of prior BCG courses was 2 (range: 1 to 8), with 38% of patients meeting the BCG unresponsive disease definition. From an efficacy standpoint, the 1- and 2-year recurrence-free survival rates were 60% and 46%, respectively, with similar recurrence rates observed for patients with CIS versus papillary-only disease. The high-grade recurrence-free survival rates in the overall cohort at 1 and 2 years were 65% and 52%, respectively:

Among patients with BCG unresponsive disease, the 2-year high-grade recurrence-free survival rates were 50% and 58% for CIS and papillary disease-alone cases, respectively:

Overall, 43/276 patients (15.6%) underwent a radical cystectomy, at a median follow-up of 11.3 months, and 11 patients (4%) had evidence of progression to muscle invasive disease. The most common side effects were frequency/urgency and dysuria, with 41% of patients reporting symptoms during treatment, but only 9.3% having symptoms that impacted the treatment schedule. There were no reported treatment-related deaths.9
Long-term follow-up of a subset of this cohort from the University of Iowa was recently published in 2023. This analysis included 97 patients (35% BCG unresponsive), with a median follow-up of 49 months. The 3-month complete response rate was 74%, with a median duration of response of 25 months. The high-grade recurrence-free survival rates were:
- 1 year: 60%
- 2 years: 50%
- 5 years: 30%
Device-assisted Administration of Intravesical Chemotherapy
TAR-200TAR-200/gemcitabine (JNJ-17000139) is a novel drug delivery system that allows for the sustained local release of gemcitabine intravesically, relying on an osmotic system as illustrated below:
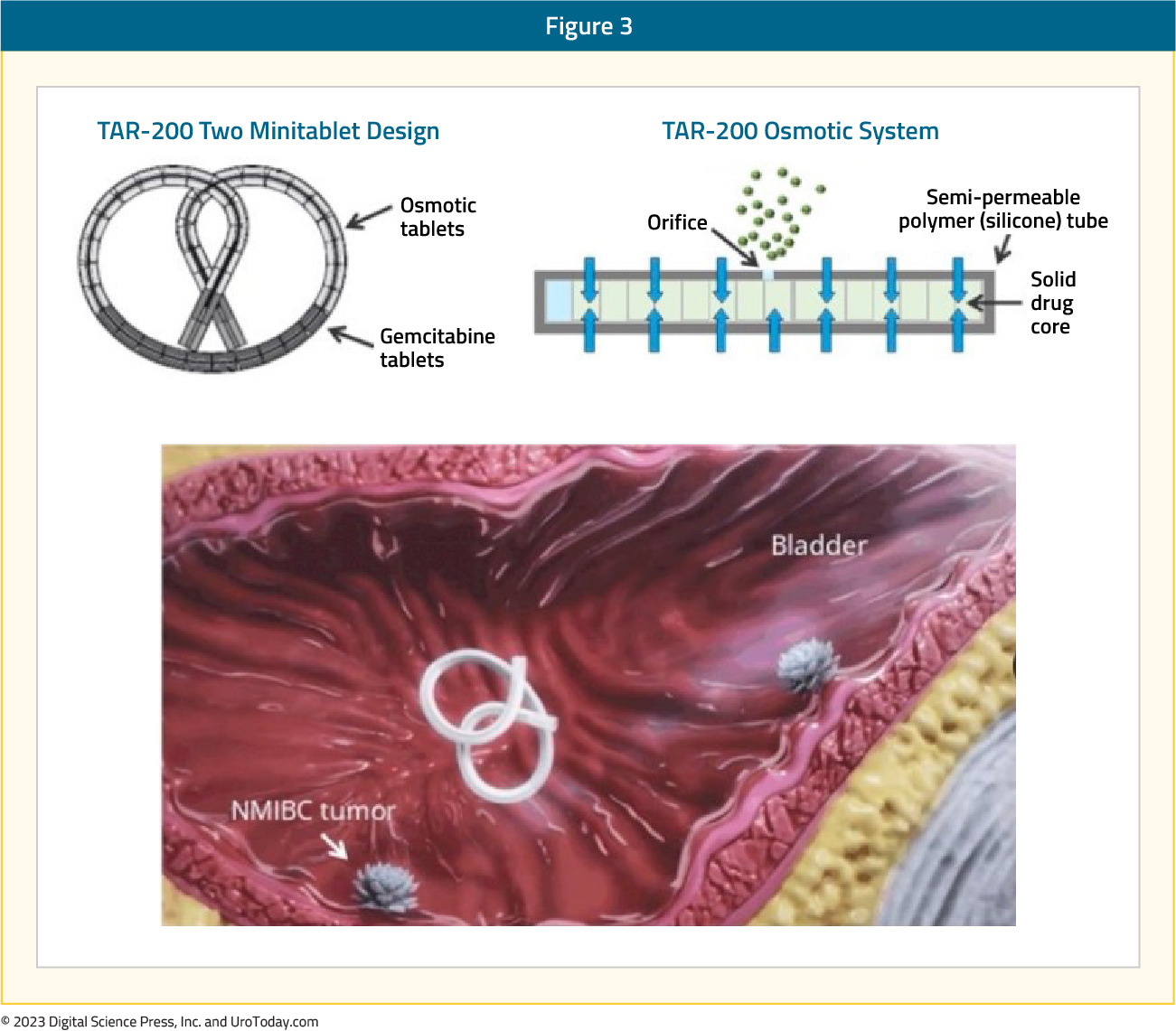
Evaluation of urine and plasma gemcitabine concentrations over a 7-day period demonstrates the ability of TAR-200 to provide sustained, local delivery of gemcitabine while limiting systemic toxicity. As demonstrated in the figure below, intravesical instillation of gemcitabine (green curve) is associated with a sharp increase/decrease in the gemcitabine urine concentration, which is non-sustained beyond day 1. Conversely, we see an increase in the urinary gemcitabine concentration between days 1 and 3 with TAR-200 (blue curve), followed by a gradual decline with measurable levels detected until at least day 7. Importantly, no gemcitabine is detected in the plasma of patients receiving TAR-200.

SunRISe-1 is a randomized trial of BCG-unresponsive, high-risk NMIBC patients with CIS +/- papillary disease, who did not receive a radical cystectomy. Patients underwent stratified randomization (by presence or absence of concomitant papillary disease) in a 2:1:1 fashion to either:
- Cohort 1: TAR-200 + cetrelimab (PD-1 inhibitor)
- Cohort 2: TAR-200 alone (target sample size 50, currently enrolled: 23)
- Cohort 3: Cetrelimab alone (target sample size 50, currently enrolled: 24)
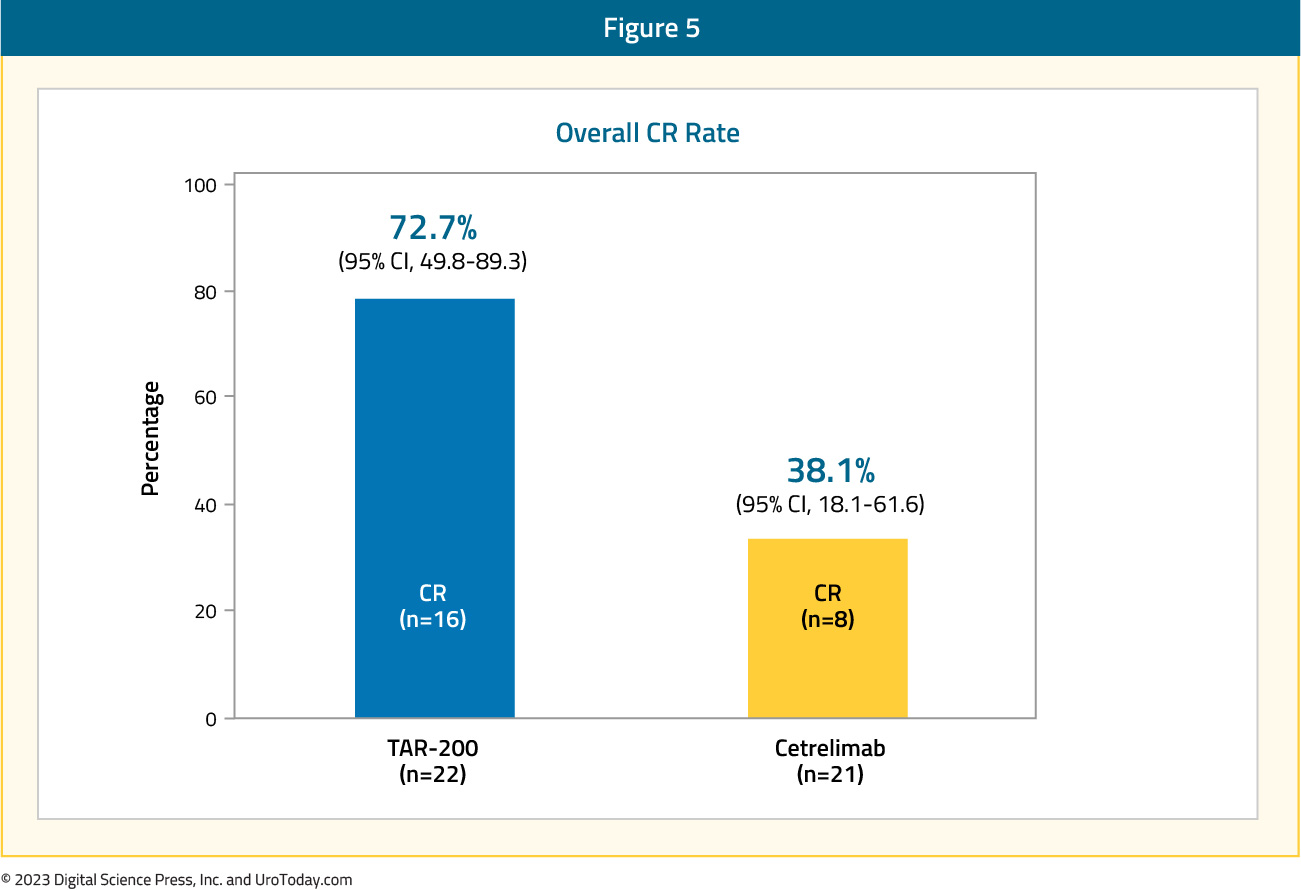
The median patient age was 70 – 72 years, pure CIS was present in 70% and 65% of patients in the TAR-200 and cetrelimab groups, respectively, and the median total doses of prior BCG were 12 in each arm. From an efficacy standpoint, 73% of patients in the TAR-200 arm achieved a complete response (median duration of response not yet reached), defined based on the results of cystoscopy, centrally assessed urine cytology, and mandated biopsy at weeks 24 and 48. The CR rate in the cetrelimab arm was 38%.
Overall, most adverse events in the TAR-200 group were grade ≤2, with those reported in the cetrelimab group similar to that expected and observed with other PD-1 agents. 9% and 4% of patients discontinued TAR-200 and cetrelimab due to treatment-related adverse events. In each arm, 1 patient had treatment-related serious adverse events and 2 patients had treatment-related grade ≥3 adverse events. No deaths were observed in the study.11

Given these promising findings from the TAR-200 monotherapy arm, Janssen announced that they will be suspending further enrollment to the TAR-200 + cetrelimab arm.
Radiofrequency-induced Thermochemotherapy
In 2009, the results of a multi-institutional analysis of 51 patients from 15 European centers receiving mitomycin combined with intravesical hyperthermia in patients with mainly BCG-failing CIS (35/51) were published. Patients received mitomycin via the Synergo® system SB-TS 101, which uses an intravesical microwave applicator to deliver hyperthermia to the bladder wall via direct irradiation, weekly for 6–8 weeks, followed by 4–6 sessions every 6–8 weeks. Of 49 evaluable patients, there was evidence of a complete CIS response (negative biopsy and cytology) at 3 months in 45 patients (92%). Patients had similar outcomes irrespective of whether they met the BCG failure definition or not. Among the 45 responders, 49% had a recurrence at a median follow-up of 22 months. The most common adverse events were bladder spasms (13.1%), pain (12.7%), and dysuria (6.2%), and were generally mild in severity.12A follow-up analysis of 150 patients who received ≥6 radiofrequency mitomycin instillations (induction and maintenance) was reported in 2018. All included patients had either pathology or cystoscopy plus cytology available at 6 months of follow-up. Of these 150 patients, 50 (33.3%) had BCG-unresponsive disease, with a 6-months complete response rate of 36% in this subgroup. The two-year recurrence-free survival rate was 82.6%, with a three-year cystectomy-free rate of 71.4%. Treatment discontinuation due to adverse events occurred in 13.4% of patients receiving induction treatment and 17.8% of those receiving maintenance instillations.13
Hyperthermic Intravesical Chemotherapy
In 2018, de Joeng et al. reported the results of hyperthermic intravesical chemotherapy (HIVEC) in 52 patients with BCG unresponsive NMIBC. Patients received intravesical instillations of mitomycin (80 mg in 50 mL of distilled water) that were extravesically heated up to 41-43°C and recirculated during 60 minutes at 200 m/min at stable pressure. All instillations were conducted with the Combat BRS system V2.0. The 3-months complete response rate was 75%. By 12 months follow-up, 47% of patients had remained disease-free. The overall median disease-free survival was 17.7 months and was significantly longer in those with papillary disease at 28.8 months versus 17.7 months in those with CIS. Patients in the ‘very high risk’ BCG unresponsive group had the shortest disease-free survival duration at 12.1 months. Any adverse event was reported in 69% of patients, with almost all grade 1-2 in severity, most common of which was urinary frequency/urgency (35%), urinary tract pain (15%), and bladder spasms (7%).14
In 2022, results of the multi-institutional Hyperthermic Chemotherapy registry (HIVEC-E) were published. These included a total of 1,028 patients, with 172 (21%) and 74 (9%) having disease classified as BCG unresponsive and failure, respectively. The 12- and 24-months recurrence-free survival rates for the BCG unresponsive cohort were 78.1% and 57.4%, respectively. Among patients in the BCG unresponsive cohort, the 24-months recurrence-free survival rates were superior for those papillary only disease (64.5% versus 43.6% for those with CIS). Minor and severe adverse events occurred in 26% and 2% of patients, respectively.15
Photodynamic Therapy
TLD-1433 is a novel ruthenium-based photosensitizer that selectively binds to bladder cancer cells. When activated by a 520 nm intravesical laser (TLC-3200) under general anesthesia (90 J/cm2 of laser light), it generates cytotoxic singlet oxygen and radical oxygen species, leading to cell death. PDT is also known to induce an antitumor cascade of immune signaling.16,17
At ASCO GU 2023, Dr. Girish Kulkarni presented the interim results of a phase II trial evaluating this intravesical photo dynamic therapy in patients with BCG unresponsive CIS. At the time of presentation, the trial had enrolled 52 patients (70% pure CIS, 20% CIS + HG T1, 10% CIS + Ta). This was a heavily pre-treated cohort, with 21% having >19 prior BCG instillations. A complete response, defined by a negative cystoscopy and cytology, was observed in 54% of patients. Among the 12 patients who had available follow-up at 450 days, 8 (67%) had a complete response. While 9/52 patients experienced a serious adverse event, none were deemed directly treatment-related per the Data Safety Monitoring Board.18
Enfortumab Vedotin
Enfortumab vedotin (EV) is a Nectin-4 targeted antibody-drug conjugate. Nectin-4 is highly expressed on bladder tumor cells, making it an attractive target for such agents. Systemic use of EV has previously demonstrated an overall survival benefit in the 3rd line setting for patients with locally advanced or metastatic urothelial carcinoma, who had previously received platinum-based therapy and a PD-1 or PD-L1 inhibitor (EV-301).19 Based on its demonstrated benefit in locally advanced/metastatic urothelial carcinoma, EV is currently being evaluated in earlier settings. EV-104 (NCT05014139) is a phase 1, open-label, multicenter, dose-escalation, and dose-expansion study of intravesical EV in adults with high-risk BCG-unresponsive NMIBC (CIS +/- papillary disease) who are ineligible for or refuse radical cystectomy. The primary study objectives are to evaluate the safety and tolerability of intravesical EV and determine the maximum-tolerated and recommended phase II doses of intravesical EV.Similar in concept to BCG and intravesical chemotherapy regimens, the EV-104 treatment regimen will include an induction phase, whereby patients will receive intravesical EV weekly for 6 weeks followed by monthly maintenance for a total of 9 additional EV doses. Patients will undergo cystoscopy and cytology every 3 months and annual upper tract imaging.

The study is currently enrolling in the United States (since January 2022) with additional sites planned in Canada and Europe, including the UK.
Erdafitinib
Erdafitinib is an oral selective pan-fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor that is approved for locally advanced or metastatic urothelial carcinoma in adults with FGFR3/2 alterations who have progressed during or after at least one line of platinum-containing chemotherapy.20 THOR-2 is a multi-cohort, phase II trial of erdafitinib in patients with high-risk NMIBC, with Cohort 2 including patients with BCG-unresponsive CIS (+/- papillary disease) and FGFR3/2 alterations. In this cohort, patients received continuous oral erdafitinib 6 mg once daily.Interim results were presented at ASCO GU 2023 by Dr. James Catto. At time of presentation, Cohort 2 had enrolled 10 patients with a median age of 72 years. The complete response rates at the 1st (Cycle 3, Day 1) and 2nd evaluations (Cycle 6, Day 1) were 100% and 75%, respectively. At a median follow-up of 10 months, the median duration of response was 3.0 months. Grade 3 or worse treatment-related adverse events occurred in 3 patients (30%), and included dry mouth, stomatitis, nail disorder, onychomadesis, acute kidney injury, chronic kidney disease, sepsis, and hypotension. One patient discontinued treatment due to adverse events. There were no treatment-related deaths.
Conclusions
Intravesical chemotherapeutic agents have emerged as promising treatment options for patients with BCG unresponsive NMIBC. Given its ease of administration and wide availability, the intravesical combination of gemcitabine plus docetaxel has emerged as one of the most commonly used treatments in this setting in clinical practice. TAR-200, via a local sustained release of gemcitabine, demonstrates excellent early response rates in SunRISE-1. These agents add to the growing armamentarium in the BCG unresponsive disease space with future regulatory approvals by the FDA contingent on further follow-up of current and future studies of these agents.
Published September 2023
- Written by: Rashid K. Sayyid, MD, MSc University of Toronto Toronto, ON and Zachary Klaassen, MD, MSc Medical College of Georgia Augusta, Georgia, USA
- References:
- EAU Guidelines: Non-muscle-invasive Bladder Cancer. https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer. Accessed on July 30, 2023.
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766-73.
- Schmidt S, Kunath F, Coles B, et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Review. 2020;1(1):CD011935.
- Babjuk M, Burger M, Comperat EM, et al. European Association of Urology Guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) - 2019 Update. Eur Urol. 2019;76(5):639–57.
- FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer. Accessed on July 30, 2023.
- FDA D.I.S.C.O. Burst Edition: FDA approval of Adstiladrin (nadofaragene firadenovec-vncg) for patients with high-risk Bacillus Calmette-Guérin unresponsive non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumors. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-adstiladrin-nadofaragene-firadenovec-vncg-patients-high-risk#:~:text=On%20December%2016%2C%202022%2C%20the,with%20or%20without%20papillary%20tumors. Access on July 30, 2023.
- Boorjian SA, Alemozaffar M, Konety BR, Shore ND, Gomella LG, Kamat AM, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021;22(1):107–117.
- O’Donnell MA, Lilli K, Leopold C, National Bacillus Calmette-Guerin/interferon phase 2 investigator G Interim results from a national multicenter phase II trial of combination bacillus Calmette-Guerin plus interferon alfa-2b for superficial bladder cancer. J Urol. 2004;172(3):888–93.
- Steinberg RL, Thomas LJ, Brooks N, et al. Multi-Institution Evaluation of Sequential Gemcitabine and Docetaxel as Rescue Therapy for Nonmuscle Invasive Bladder Cancer. J Urol. 2020;203(5):902-9.
- Chevuru PT, McElree IM, Mott SL, et al. Long-term follow-up of sequential intravesical gemcitabine and docetaxel salvage therapy for non-muscle invasive bladder cancer. Urol Oncol. 2023;41(3):148.e1-7.
- Daneshmand S, van der Heijden MS, Jacob JM, et al. LBA02-03 FIRST RESULTS FROM SunRISE-1 IN PATIENTS WITH BCG UNRESPONSIVE HIGH-RISK NON–MUSCLE-INVASIVE BLADDER CANCER RECEIVING TAR-200 IN COMBINATION WITH CETRELIMAB, TAR-200, OR CETRELIMAB ALONE. J Urol. 2023;209(Suppl 4):e1187.
- Witjes JA, Hendricksen K, Gofrit O, Risi O, Nativ O. Intravesical hyperthermia and mitomycin-C for carcinoma in situ of the urinary bladder: experience of the European Synergo® working party. World J Urol. 2009;27:319-24.
- Van Valenberg FJP, Kajtazovic A, Canepa G, et al. Intravesical Radiofrequency-Induced Chemohyperthermia for Carcinoma in Situ of the Urinary Bladder: A Retrospective Multicentre Study. Bladder Cancer. 2018;4(4):365-76.
- De Jong JJ, Hendricksen K, Rosier M, Mostafid H, Boormans JL. Hyperthermic Intravesical Chemotherapy for BCG Unresponsive Non-Muscle Invasive Bladder Cancer Patients. Bladder Cancer. 2018;4(4):395-401.
- Tan WP, Plata Bello A, Garcia Alvarez C, et al. A Multicenter Study of 2-year Outcomes Following Hyperthermia Therapy with Mitomycin C in Treating Non-Muscle Invasive Bladder Cancer: HIVEC-E. Bladder Cancer. 2022;8(4):379-93.
- Alzeibak R, Mishchenko TA, Shilyagina NY, et al. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer. 2021;9:e001926.
- Meng Z, Zhou X, Xu J, et al. Light-triggered in situ gelation to enable robust photodynamic-immunotherapy by repeated stimulations. Adv Mater. 2019;31:e1900927.
- Kulkarni GS, Richards KA, Black PC, et al. A phase II clinical study of intravesical photo dynamic therapy in patients with BCG-unresponsive NMIBC (interim analysis). J Clin Oncol. 2023;6(Suppl):528.
- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021;384:1125-35.
- Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2019;381:338-48.
Novel Treatment Options for BCG Naïve Non-Muscle Invasive Bladder Cancer: Immune Priming and Immune Check Point Inhibitors
Introduction
Immune checkpoint inhibitors have emerged as a guideline-recommended first line treatment option for patients with cisplatin-ineligible, metastatic urothelial carcinoma of the bladder and as second line therapy for patients with metastatic disease progressing during, or after, platinum-based combination chemotherapy.1 Pembrolizumab, a Programmed Death-1 (PD-1) inhibitor, has been recently approved by the US Food and Drug Administration for the treatment of patients with Bacillus Calmette Guerin (BCG)-resistant non-muscle invasive bladder cancer (NMIBC), based on the results of the KEYNOTE-057 trial.2,3
Given that patients with NMIBC receiving adjuvant BCG post-TURBT have estimated risks of disease recurrence and progression of 40% and 10%, respectively,4 the BCG naïve NMIBC space may provide an opportunity to move these agents up even further along the bladder cancer disease spectrum. In this Center of Excellence article, we will summarize the current state of the evidence for ongoing trials evaluating immune check point inhibitors and other immune priming interventions in combination with BCG for the treatment of BCG naïve NMIBC.
Pembrolizumab + BCG
Previous studies have demonstrated that programmed cell death ligand 1 (PD-L1) expression is significantly increased in BCG-induced bladder granulomata of patients with BCG unresponsive disease.5 As such, it has been hypothesized that the addition of a PD-1 inhibitor, such as pembrolizumab, may overcome this potential underlying mechanism of resistance.
The combination of pembrolizumab + BCG has previously been evaluated in the setting of a phase I trial for patients with BCG unresponsive NMIBC. This combination was determined to be relatively safe, and the 13 evaluable patients had a 3-month complete response rate of 69%.6
KEYNOTE-676 is a randomized, comparator-controlled trial evaluating the efficacy and safety of pembrolizumab + BCG in patients with high-risk NMIBC (T1, CIS, high grade Ta) who underwent cystoscopy/transurethral resection of bladder tumor ≤12 weeks before randomization and had not received BCG within the preceding two years. Patients will be randomly assigned 1:1:1 to receive:
- Pembrolizumab 400 mg IV every 6 weeks + BCG reduced maintenance (≤ 6 months)
- Pembrolizumab 400 mg IV every 6 weeks + BCG full maintenance (≤ 18 months)
- BCG monotherapy with BCG full maintenance
The trial schema for KEYNOTE-676 cohort B is as follows:
The primary endpoint for this trial is event-free survival, defined as the time from random assignment to the first occurrence of any of the following:
- High-grade Ta, CIS, or any T1 disease of the bladder
- High-risk disease (high-grade Ta, CIS, or ≥T1) of the urethra or upper tract
- Locally advanced/metastatic disease determined by blinded independent central review
- Death from any cause
The secondary endpoints will include complete response rate by blinded independent central review, duration of response, disease-specific survival, time to cystectomy, overall survival, and safety.7 As follows is a geographical representation of the countries currently enrolling patients in KEYNOTE-676:
Additionally, a single arm, phase II trial (NCT03504163) from the Memorial Sloan Kettering Cancer Center is evaluating the combination of BCG + pembrolizumab in patients with high-risk T1 bladder cancer, with an additional exploratory cohort of patients with upper tract disease. This study will plan to enroll 37 patients, who will receive pembrolizumab 400 mg IV at 6-week intervals (total 9 doses), with BCG (TICE strain, 50 mg) administered once weekly for 6 weeks as induction, followed by maintenance consistent with standard clinical practice. BCG will be started on week 3 after the first infusion of pembrolizumab to allow for the initial priming of T cells to further enhance the effects of BCG treatment. The primary outcome is the proportion of patients who remain free of high-grade disease recurrences at 6 months post-treatment initiation.8
Atezolizumab + BCG
BladderGATEBladderGATE (NCT04134000) is a phase Ib-II trial evaluating the safety and efficacy of atezolizumab (anti-PD-L1) + BCG in patients with high-risk NMIBC, who are either BCG-naïve or had not received BCG in the preceding two years. Patients in this trial will receive either:
- Induction BCG with 1 instillation every week + IV atezolizumab 1,200 mg every 3 weeks (Dose level 0)
- Induction BCG with ½ instillation every week + IV atezolizumab 1,200 mg every 3 weeks (Dose level -1)
Following induction, BCG will be administered at weeks 13-15, 25-27, and 49-51, with atezolizumab concurrently administered for up to 1 year. Patients were accrued to each dose level in cohorts of 10 patients until the maximum tolerated dose is achieved (dose at which < 4 out of 10 patients experience dose-limiting toxicity). The interim safety results were recently presented at ASCO 2023. This analysis included 34 patients, with no dose-limiting toxicities reported in the first 10 patients included at dose level 0. The most frequent grade 3-4 adverse events were:
- Asthenia (9%)
- Myocarditis (3%)
- Immune-mediated hepatitis (3%)
- Hyponatremia (3%)
- Encephalopathy (3%)
- Guillain-Barre syndrome (3%)
Two patients discontinued the study treatment due to immune-mediated Grade 3 hepatitis and pneumonitis, respectively.9
ALBANALBAN (AFU-GETUG 37; NCT03799835) is a phase III trial across 30 centers in France evaluating the efficacy and safety of atezolizumab given in combination with BCG versus BCG alone in patients with BCG naïve, high-risk NMIBC (T1, high-grade, and/or CIS). Eligible patients will be randomized 1:1 to:
- Arm A: BCG alone with six weeks induction followed by three weekly maintenance instillations at 3, 6, and 12 months
- Arm B: BCG + atezolizumab (1,200 mg IV every 3 weeks for up to 1 year)
The primary endpoint is recurrence-free survival in the intent-to-treat population, with secondary efficacy endpoints of overall survival, progression-free survival, complete response, disease worsening, quality of life, and safety outcomes. Study enrollment began in December 2018 with a target of 614 patients.10
Durvalumab + BCG
POTOMAC (NCT03528694) is an open label, multicenter, randomized trial evaluating the combination of durvalumab (anti-PD-L1) and BCG in BCG-naïve patients with high-risk NMIBC (any high-grade disease, T1, CIS, LG Ta if >3 cm, recurrent, and multifocal). This trial will randomize 1,018 patients to:
- BCG induction + maintenance for 24 months
- BCG induction + maintenance + durvalumab (1,500 mg every 4 weeks for 13 cycles)
- BCG induction only (no maintenance) + durvalumab
The study design is as follows:
The primary study endpoint is disease-free survival, with secondary endpoints including the proportion of patients alive and disease-free at 24 months, 5-year overall survival, pharmacokinetics, immunogenicity, safety, tolerability, and health-related quality of life.11
Sasanlimab + BCG
Sasanlimab is an anti-PD-1 monoclonal antibody that has demonstrated an acceptable safety profile and promising clinical activity in patients with locally advanced or metastatic urothelial carcinoma, within the context of a phase 1 trial.12 The phase 3 CREST study (NCT04165317) Cohort A will evaluate subcutaneous injection sasanlimab in patients with BCG naive NMIBC. Patients in this cohort will be randomized to one of three arms:
- Arm A: Sasanlimab + BCG induction + maintenance
- Arm B: Sasanlimab + BCG induction only
- Arm C: BCG induction + maintenance
This trial will assess for between-arm differences in event-free, disease-specific, and overall survivals, complete response rate, adverse events/safety profile, and health-related quality of life.13 Of note, On August 31, 2022, the Sponsor announced the discontinuation of enrollment to Part B (Cohort B), which enrolled participants with BCG unresponsive NMIBC. The decision to discontinue enrollment to Part B (Cohort B) was not made for safety reasons.14
Immune Priming: Intradermal BCG
The PRIME trial (SWOG S1612) is evaluating whether intradermal BCG inoculation may potentiate the immune effects of subsequent intravesical BCG instillations. This hypothesis was recently tested in a single arm trial that demonstrated that percutaneous BCG administered 21 days prior to intravesical instillation in patients with high-risk NMIBC boosted BCG-specific immunity at 3 months and increased the activation status of in vitro expanded circulating NK and γδ T cells and their cytotoxicity against bladder cancer cells.15
In the PRIME trial, the Tokyo BCG strain is being used for both intradermal inoculation and intravesical instillation in a three-arm design with TICE strain BCG also used as a standard of care comparator. This study was designed to test:
- The comparitive superiority between intravesical Tokyo strain BCG when combined with intradermal inoculation as compared to intravesical alone (arms 2 and 3)
- The non-inferiority of intravesical Toyko strain alone to intravesical TICE strain (arms 1 and 2).
- TICE strain is currently the only strain that is FDA approved and in production in the USA (Armond-Frappier and Connaught are also FDA approved, but not currently in production). As such, if the Tokyo strain is found to be non-inferior to TICE, this may facilitate its subsequent FDA approval and increased the availability of additional BCG strains during the current shortage
The study design is as follows:
This trial has now fully accrued and is awaiting readout in the nearby future.
Conclusions
Numerous ongoing trials are evaluating the combination of BCG and an immune checkpoint inhibitor for patients with BCG naïve NMIBC. By inhibiting the PD-1/PD-L1 axis, it is hypothesized that these agents may help overcome underlying mechanisms of resistance inherent to BCG-resistant strains, and thus improve on the historic recurrence and progression rates observed with BCG treatment alone. Many of these trials have completed accrual, and we await the results of these combinatory trials in the upcoming years.
- Written by: Rashid K. Sayyid, MD, MSc, University of Toronto, Toronto, ON & Zachary Klaassen, MD, MSc, Medical College of Georgia, Augusta, Georgia, USA
- References:
- EAU Guidelines: Non-muscle-invasive Bladder Cancer. https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer. Accessed on Aug 6, 2023.
- FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer. Accessed on Aug 6, 2023.
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22(7):919-30.
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766-73.
- Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7–H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109(8):1499-505
- Alanee S, Sana S, El-Zawahry A, et al. Phase I trial of intravesical Bacillus Calmette-Guérin combined with intravenous pembrolizumab in recurrent or persistent high-grade non-muscle-invasive bladder cancer after previous Bacillus Calmette-Guérin treatment. World J Urol. 2021;39(10):3807-13.
- Kamat AM, Shariat S, Steinberg GD, et al. Randomized comparator-controlled study evaluating efficacy and safety of pembrolizumab plus Bacillus Calmette-Guérin (BCG) in patients with high-risk nonmuscle-invasive bladder cancer (HR NMIBC): KEYNOTE-676 cohort B. J Clin Oncol. 2022;40(Suppl 6): TPS597
- ClinicalTrials.gov - Pembrolizumab (MK-3475) and Bacillus Calmette-Guérin (BCG) as First-Line Treatment for High-Risk T1 Non-Muscle-Invasive Bladder Cancer (NMIBC) and High-Grade Non-Muscle-Invasive Upper Tract Urothelial Carcinoma (NMI-UTUC)].
- Castellano D, de Velasco G, Carretero-Gonzalez A, et al. Atezolizumab + intravesical BCG (bacillus Calmette-Guerin) upfront combination in high risk non–muscle- invasive bladder cancer (NMIBC) patients: Safety interim report of BladderGATE phase I-II study. J Clin Oncol. 2023;41(Supp 16):e16590.
- Roupret M, Neuzillet Y, Bertaut A, et al. ALBAN: An open label, randomized, phase III trial, evaluating efficacy of atezolizumab in addition to one year BCG (Bacillus Calmette-Guerin) bladder instillation in BCG-naive patients with high-risk nonmuscle invasive bladder cancer (AFU-GETUG 37). J Clin Oncol. 2019;37(Suppl 15):TPS4589.
- De Santis M, Abdrashitov R, Hegele A, et al. A phase III, randomized, open-label, multicenter, global study of durvalumab and bacillus calmette-guérin (BCG) versus BCG alone in high-risk, BCG-naïve non-muscle-invasive bladder cancer (NMIBC) patients (POTOMAC). J Clin Oncol. 2019;37(Suppl 7):TPS500.
- Shore ND, Powles T, Bedke J, et al. A phase 3 study of the subcutaneous programmed cell death protein 1 inhibitor sasanlimab as single agent for patients with bacillus Calmette-Guérin, unresponsiv,e high-risk, non-muscle invasive bladder cancer: CREST Study Cohort B. J Clin Oncol. 2022;40(Suppl 16):40.
- ClinicalTrials.gov. A Study of Sasanlimab in People With Non-muscle Invasive Bladder Cancer (CREST). https://classic.clinicaltrials.gov/ct2/show/NCT04165317. Access on Aug 6, 2023.
-
Clinicaltrials.gov. Available at: https://www.clinicaltrials.gov/study/NCT04165317?intr=sasanlimab&rank=8 (Accessed: 26 April 2024)
Novel Treatment Options for BCG Naïve Non-Muscle Invasive Bladder Cancer: Intravesical Chemotherapy
Introduction
Bacillus Calmette Guerin (BCG) is currently guideline-recommended in the adjuvant setting for patients with intermediate or high-risk non-muscle invasive bladder cancer (NMIBC).1 This is based on the results of numerous randomized clinical trials and meta-analyses demonstrating its ability to reduce the rates of disease recurrence and progression, compared to transurethral resection of bladder tumor (TURBT) alone or other adjuvant therapies.2-5
- Written by: Rashid K. Sayyid, MD, MSc University of Toronto Toronto, ON & Zachary Klaassen, MD, MSc Medical College of Georgia Augusta, Georgia, USA
- References:
- EAU Guidelines: Non-muscle-invasive Bladder Cancer. https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer. Accessed on Aug 5, 2023.
- Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC genito-urinary group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guerin, and bacillus Calmette-Guerin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57(5):766-73.
- Schmidt S, Kunath F, Coles B, et al. Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer. Cochrane Database Syst Review. 2020;1(1):CD011935.
- Malmstrom PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guerin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56(2):247-56.
- Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169(1):90-5.
- Oresta B, et al. Sci Transl Med. Jan 6;13(575):eaba6110 Clinical trial information: EudraCT 2021-003751-42_studio ICH-013 (MMC).
- Di Stasi SM, Giannantoni A, Giurioli A, et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: a randomised controlled trial. Lancet Oncol. 2006;7:43-51.
- Solsona E, Madero R, Chantada V, et al. Sequential combination of mitomycin C plus bacillus Calmette-Guérin (BCG) is more effective but more toxic than BCG alone in patients with non-muscle-invasive bladder cancer in intermediate- and high-risk patients: final outcome of CUETO 93009, a randomized prospective trial. Eur Urol. 2015;67(3):508-16.
- Kaasinen E, Wijkstrom H, Rintala E, et al. Seventeen-year follow-up of the prospective randomized Nordic CIS study: BCG monotherapy versus alternating therapy with mitomycin C and BCG in patients with carcinoma in situ of the urinary bladder. Scand J Urol. 2016;50(5):360-8.
- De Nunzio C, Leonardo C, Carbone A, et al. MP63-12 THE EFFECTS OF SEQUENTIAL MITOMYCIN AND BACILLUS CALMETTE-GUÉRIN TREATMENT VERSUS BACILLUS CALMETTE-GUÉRIN MONOTHERAPY IN PATIENTS WITH HIGH-RISK NON-MUSCLE INVASIVE BLADDER CANCER: MITO-BCG (EUDRACT-2017-004540-37). J Urol. 2023;209(Suppl 4):e876.
- Jagannath C, Lindsey DR, Dhandayuthapani S, et al.. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–76.
- Ji N, Mukherjee N, Reyes RM, et al. Rapamycin enhances BCG-specific γδ T cells during intravesical BCG therapy for non-muscle invasive bladder cancer: a randomized, double-blind study. J Immunother Cancer. 2021;9(3):e001941.
- Steinberg RL, Thomas LJ, Brooks N, et al. Multi-Institution Evaluation of Sequential Gemcitabine and Docetaxel as Rescue Therapy for Nonmuscle Invasive Bladder Cancer. J Urol. 2020;203(5):902-9.
- McElree IM, Steinberg RL, Mott SL, et al. Comparison of Sequential Intravesical Gemcitabine and Docetaxel vs Bacillus Calmette-Guérin for the Treatment of Patients With High-Risk Non–Muscle-Invasive Bladder Cancer. JAMA Netw Open. 2023;6(2):e230849.
- Guerrero-Ramos F, Gonzalez-Padilla DA, Gonzalez-Diaz A, et al. Recirculating hyperthermic intravesical chemotherapy with mitomycin C (HIVEC) versus BCG in high-risk non-muscle-invasive bladder cancer: results of the HIVEC-HR randomized clinical trial. World J Urol. 2022;40(4):999-1004.
Mental Health in Bladder Cancer Patients: Clinical Implications and Outcomes
Introduction
In 2021 in the United States, there will be approximately 83,730 new cases of bladder cancer (~64,280 men and 19,450 women) and approximately 17,200 deaths from bladder cancer (12,260 men and 4,940 women). On a global scale, in 2017 it was estimated that there were 2.63 million (95% CI 2.57-2.72 million) bladder cancer cases, involving 2.03 million (95% CI 1.96-2.11 million) men and 0.60 million (95% CI 0.58-0.62 million) women.1 As such, although bladder cancer may be a lethal diagnosis for some, there are also millions of bladder cancer survivors worldwide. Bladder cancer patients, generally, have a higher level of comorbidity than most other patients with genitourinary malignancies, and recent literature over the last 5 years or so suggests that bladder cancer patients have proportionately worse depression and mental health, as well as being at increased risk of suicidal death when compared to the general population. This article will discuss the impact of depression and mental health associated with a bladder cancer diagnosis, assess the impact of a bladder cancer diagnosis on risk of suicide, and discuss future endeavors and areas of focus for improving outcomes for patients with bladder cancer.
Depression and Anxiety
In Western countries, the lifetime prevalence of major depression is estimated at 16.5%. Work from >30 years ago from the Psychological Collaborative Oncology Group suggested that 47% of adult patients with cancer were maladjusted to an illness crisis, with the most common manifestation being adjustment disorder with depression. In 2018, Vartolomei and colleagues2 performed a systematic review of the literature assessing the prevalence of depression and anxiety among patients with bladder cancer, including 13 studies encompassing 1,659 patients. Six studies assessed depression prior and after treatment at 1, 6, and 12 months, whereas four studies investigated anxiety, and seven additional studies reported the prevalence of depression and anxiety among patients with bladder cancer at a specific time-point. Overall, pretreatment depression rates ranged from 5.7 to 23.1% and post-treatment from 4.7 to 78%, while post-treatment anxiety rates ranged from 12.5 to 71.3%.
Compared to the prostate cancer literature, there is a relative paucity of data assessing how specific aspects of treatment may affect depression scores amongst bladder cancer patients. In a single-center setting, Zhang et al.3 evaluated anxiety, depression, and quality of life by patients' self-reported scales, as well as predictive factors for anxiety and depression exacerbation among 194 muscle-invasive bladder cancer patients receiving adjuvant chemotherapy after radical cystectomy. The Hospital Anxiety and Depression Scale (HADS) was used to evaluate anxiety and depression, and the EORTC QLQ-C30 Scale was used to assess quality of life. After adjuvant chemotherapy, this study found that HADS-Anxiety score (p = 0.042), anxiety percentage (p = 0.036), HADS-Depression score (p < 0.001), depression percentage (p = 0.002) and the EORTC QLQ-C30 Functional score (p = 0.002) were increased compared with baseline. Furthermore, on multivariable analysis, increasing age (p < 0.001), increasing BMI (P = 0.021) and hypertension (P = 0.001) were associated with worsening of the HADS-Anxiety score, while male gender (P < 0.001) was associated with worsening of HADS-Depression score during adjuvant chemotherapy.
Taken together, given the prevalence of bladder cancer and the associated post-diagnosis/treatment depression and anxiety that occurs, this is an actionable patient population for targeting psycho-oncology intervention, particularly in the comorbid, elderly, and male patients that are particularly at risk of depression or anxiety.
Broader Mental Health Considerations
Although the majority of bladder cancer literature has been dedicated to optimizing oncological outcomes and focuses on physical prognostic criteria, emerging data have suggested that both pre-and post-treatment mental health (not just isolated to depression) may play as important a role in patient outcomes as physical health. In a systematic review assessing the prevalence and impact of mental health disorders in bladder cancer patients, Pham et al.4 identified 87 publications that met initial inclusion criteria, leading to 19 relevant publications incorporated into the review, of which 11 were prospective studies and 8 were retrospective studies. They found that mental health issues, such as depression and anxiety, often coexist with a diagnosis of bladder cancer. Further, those with a worse oncologic prognosis have a greater psychological burden. Additionally, poor mental health was associated with adverse treatment outcomes such as postsurgical complication rates and survival outcomes.
A similar study to characterize the patterns of care and survival of elderly patients with a pre-existing mental illness diagnosed with bladder cancer was undertaken by Sathianathen et al.5 using the SEER-Medicare database. This study included elderly patients (≥68 years old) with localized bladder cancer from 2004 to 2011, stratified by the presence of a pre-existing mental illness at the time of cancer diagnosis: severe mental illness (consisting of bipolar disorder, schizophrenia, and other psychotic disorders), anxiety, and/or depression. The authors examined
the stage at presentation and receipt of guideline-concordant therapies (ie. radical cystectomy for muscle-invasive disease). Among 66,476 patients meeting inclusion criteria, 6.7% (n = 4,468) had a pre-existing mental health disorder at the time of cancer diagnosis. These patients were significantly more likely to present with muscle-invasive disease than those with no psychiatric history (23.0% vs 19.4%, p < 0.01). In patients with muscle-invasive disease, those with severe mental illness (OR 0.55, 95% CI 0.37-0.81) and depression only (OR 0.71, 95% CI 0.58-0.88) were significantly less likely to undergo radical cystectomy or trimodality therapy. However, patients in this subgroup who underwent radical cystectomy had significantly superior overall (HR 0.54, 95% CI 0.43-0.67) and disease-specific survival (HR 0.76, 95% CI 0.58-0.99) compared with those who did not receive curative treatment.
Pre-cancer diagnosis utilization of psychiatric resources has been suggested as a more accurate assessment of mental health comorbidity burden at the population level rather than relying on specific ICD-9/ICD-10 codes for mental health illnesses. To assess this impact in a Canadian health care setting, Klaassen et al.6 included all residents of Ontario diagnosed with one of the ten most prevalent malignancies (which included bladder cancer) from 1997 to 2014. A psychiatric utilization grade (PUG) score in the five years prior to a cancer diagnosis was calculated as follows: 0 – none; 1 – outpatient psychiatric utilization; 2 - emergency department psychiatric utilization; and 3 – psychiatric specific hospital admission. A total of 676,125 patients were included, specifically 359,465 (53.2%) with PUG score 0, 304,559 (45.0%) with PUG score 1, 7,901 (1.2%) with PUG score 2, and 4,200 (0.6%) with PUG score 3. Increasing PUG score was independently associated with worse cancer-specific morality, with an effect gradient across the intensity of pre-diagnosis psychiatric utilization (vs PUG score 0): PUG score 1 HR 1.05 (95% CI 1.04-1.06), PUG score 2 HR 1.36 (95% CI 1.30-1.42), and PUG score 3 HR 1.73 (95% CI 1.63-1.84). In a subgroup analysis specific to anatomic site, bladder cancer patients with pre-diagnosis psychiatric utilization of resources worse cancer-specific morality with increasing PUG score (vs PUG score 0): PUG score 1 HR 1.09 (95% CI 1.03-1.14), PUG score 2 HR 1.29 (95% CI 1.02-1.64), and PUG score 3 HR 2.18 (95% CI 1.62-2.93).
Several studies among bladder cancer patients have also assessed the impact of post-diagnosis mental health diagnosis on outcomes and survival. Using the SEER-Medicare database from 2002 to 2011, Jazzar and colleagues7 identified 3,709 patients who were diagnosed with clinical stage T2 through T4a bladder cancer of which 1,870 (50.4%) were diagnosed with posttreatment psychiatric disorders. Patients who underwent radical cystectomy were identified as being at significantly greater risk of having a posttreatment psychiatric illness compared with those who received radiotherapy and/or chemotherapy (HR 1.19, 95% CI 1.07-1.31):

Furthermore, in adjusted analyses, diagnosis of a psychiatric disorder resulted in significantly worse overall survival (HR 2.80, 95% CI, 2.47-3.17) and cancer-specific survival (HR 2.39, 95% CI, 2.05-2.78).
This same group of investigators also used the SEER-Medicare database to assess prescription patterns and predictors in older patients with bladder cancer.8 This cohort comprised 10,516 patients diagnosed with clinical stage T1-T4a, N0, M0 bladder urothelial carcinoma from 2008 to 2012 of which 5,621 (53%) were prescribed psychotropic drugs following bladder cancer diagnosis. Overall, 3,972 (38%) patients had previous psychotropic prescriptions prior to cancer diagnosis, and these patients were much more likely to receive a post-cancer diagnosis prescription. Additionally, prescription rates for psychotropic medications were higher among patients with higher stage bladder cancer (p < 0.001). Gamma-aminobutyric acid modulators/stimulators and serotonin reuptake inhibitors/stimulators were the highest prescribed psychotropic drugs in 21% of all patients. Furthermore, adherence for all drugs was 32% at three months and continued to decrease over time.
Recent work from Ontario has also delineated the rate of post-curative intent cystectomy/radiotherapy utilization of mental health services. Using the Ontario Cancer Registry (2004-2013) to identify 4,296 patients that underwent radical cystectomy (n = 3,332) or curative radiotherapy (n = 964), Raphael et al.9 assessed mental health service use (defined as a visit to a general practitioner, psychiatrist, emergency department or hospitalization), specifically assessing baseline, peri-treatment, and post-treatment mental health service use. Compared to baseline, the rate of mental health service use was higher in the peri-treatment (aRR 1.64, 95% CI 1.48-1.82) and post-treatment periods (aRR 1.45, 95%CI 1.30-1.63), and by 2-years post-treatment, 24.6% (95% CI 23.4%-25.9%) of all patients had utilized mental health services:

Patients with baseline mental health service use had substantially higher mental health service use in the peri-treatment (aRR 5.77, 95% CI 4.86-6.86) and post-treatment periods (aRR 4.58, 95% CI 3.78-5.55). Additionally, female patients had higher use of mental health services overall, but males had a higher incremental increase in the post-treatment period compared to baseline.
Over the last several years, population-level studies have assessed the impact of pre-and post-bladder diagnosis mental health illness. Elderly patients with muscle-invasive bladder cancer and a pre-existing mental disorder are less likely to receive guideline-concordant management, which leads to poor overall and disease-specific survival. Furthermore,
half of bladder cancer patients with muscle-invasive bladder cancer who undergo treatment are subsequently diagnosed with a psychiatric disorder, resulting in worse survival outcomes compared with patients who do not have a posttreatment psychiatric diagnosis. Over half of these patients receive a psychotropic prescription within two years of their cancer diagnosis, however there appears to be low adherence to medication use, which emphasizes prolonged patient monitoring and further investigation.
Suicide
Globally, nearly 800,000 people die of suicide every year, accounting for 1.4% of deaths worldwide. Over the last decade, there have been several studies noting that suicide rates among cancer patients appear to be higher than the general population,10 including patients with genitourinary malignancies.11 Among cancer patients, patients with bladder cancer have one of the highest suicide rates. In the SEER database, over a 40-year time frame (1973-2013), 794 patients with bladder cancer (0.24%) died of suicide, 190,734 patients (57.2%) died from other causes, and 142,151 patients (42.6%) were alive.12 Significant factors associated with suicide included being unmarried (vs married: HR 1.74, 95% CI 1.49-2.04), white race (vs black: HR 2.22, 95% CI 1.32-3.74), male (vs female: HR 6.91, 95% CI 5.04-9.47), have regional disease (vs. localized: HR 2.49: 2.05-3.03), live in the Southeast United States (vs. Northeast: HR 2.43, 95% CI 1.78-3.32), not undergo a radical cystectomy (vs cystectomy: HR 1.42, 95% CI 1.03-1.94), and increasing age (>= 80 years vs 60-69 years: HR 1.32, 95% CI 1.06-1.66). As follows are suicide rates per 100,000 person-years of follow-up by a decade of bladder cancer diagnosis:

Guo et al.13 recently published a systematic review to assess how bladder cancer increases suicide risk and to identify demographic and clinical factors associated with suicidal death. This review identified five retrospective cohorts comprising 563,680 patients with bladder cancer. Higher risk of suicide by 1.90-fold was observed among patients with bladder cancer (HR 1.90, 95% CI 1.29-2.81, p = 0.001, I2 = 81.2%), especially in patients older than 70 years of age (HR 1.36, 95% CI 1.29-1.43, p < 0.00, I2 = 0%), those that are unmarried (HR 1.72, 95% CI 1.61-1.83, p < 0.001, I2 = 0%), and those with regional bladder cancer (HR = 1.88, 95% CI: 1.10-3.21; P = 0.021; I2 = 96.3%), compared to those without bladder cancer. In this systematic review, gender and race were not associated with increased suicide risk among patients with bladder cancer.
Despite the plethora of population-level studies (>20) to date suggesting an increased risk of suicidal death among cancer patients compared to the general population, all have failed to account for psychiatric care/psychiatric comorbidities before a cancer diagnosis, which may confound this relationship. In order to assess this discrepancy, Klaassen et al.14 assessed the effect of a cancer diagnosis on the risk of suicide, accounting for pre-diagnosis psychiatric care utilization using population-level data from Ontario for the ten most prevalent cancer types. As previously mentioned, a PUG score in the five years prior to a cancer diagnosis was calculated as follows: 0 – none; 1 – outpatient psychiatric utilization; 2 - emergency department psychiatric utilization; and 3 – psychiatric specific hospital admission. Noncancer controls were matched 4:1 based on sociodemographics, including the PUG score, and a marginal, cause-specific hazard model was used to assess the effect of cancer on the risk of suicidal death. Among 676,470 patients with cancer and 2,152,682 matched noncancer controls, there were 8.2 and 11.4 suicides per 1000 person-years of follow-up, respectively. Patients with cancer had an overall higher risk of suicidal death compared with matched patients without cancer (HR 1.34, 95% CI, 1.22-1.48). This effect was pronounced in the first 50 months after cancer diagnosis (HR 1.60; 95% CI, 1.42-1.81), whereas patients with cancer did not demonstrate an increased risk thereafter:

Furthermore, among individuals with a PUG score of 0 or 1, those with cancer were significantly more likely to die of suicide compared with controls. There was no difference in suicide risk between patients with cancer and controls for those who had a PUG score of 2 or 3, suggesting that among patients with severe psychiatric comorbidities the impact of a cancer diagnosis was less likely to increase risk of suicidal death. When specifically assessing bladder cancer patients versus non-cancer controls, the risk of suicidal death (accounting for pre-diagnosis psychiatric utilization of resources) was significantly higher (HR 1.73, 95% CI 1.14-2.62), with only lung cancer (HR 2.49, 95% CI 1.98-3.13) and oral cancer (HR 2.55, 95% CI 1.59-4.12) having a higher risk of suicidal death.
Bladder cancer patients have approximately a 70% increased risk of suicidal death compared to the general population/non-cancer controls. This increased risk is particularly pronounced among those that are male, elderly, white, unmarried, and those with non-localized disease. As such, early psychological support must be provided during the follow-up period of these special populations, as they may benefit from targeted survivorship plans.
Future Endeavors
Given the aforementioned data regarding the impact of depression, mental illness, and risk of suicide among bladder cancer patients, the time for prospective intervention and assessment of intervention efficacy among these patients is now.15 Bessa et al.16 performed a systematic review as part of the Medical Research Council Framework for developing complex interventions, providing an overview of the published mental wellbeing interventions that could be used to design an intervention specific for bladder cancer patients. A total of 15,094 records were collected from the search and 10 studies matched the inclusion and exclusion criteria. Of these, nine interventions were for patients with prostate cancer and one for patients with kidney cancer; no studies were found for other urological cancers. Depression was the most commonly reported endpoint measured, and of the included studies with positive efficacy, three were group interventions and two were couple interventions. In the group interventions, all studies showed a reduction in depressive symptoms, and in the couple interventions, there was a reduction in depressive symptoms and a favorable relationship cohesion.
Patient education and rehabilitation programs have also been tested prospectively among bladder cancer patients. Li et al.17 assessed the impact of this program on anxiety, depression, and quality of life in 130 muscle-invasive bladder cancer patients undergoing adjuvant chemotherapy. Patients were randomized 1:1 to the patient education and rehabilitation program group and to the control group. HADS anxiety and depression scores and QLQ-C30 scores were assessed before treatment and after treatment (week 16). They found that after 16 weeks of treatment the patient education and rehabilitation program group exhibited decreased HADS anxiety score (p = 0.036), ΔHADS anxiety score (between week 16 and week 0) (p < 0.001), and percentage of anxiety patients (p = 0.019) compared to control group. With regards to depression outcomes, the patient education and rehabilitation program group presented with numerically reduced HADS depression score (p = 0.076) compared to control group, as well as lower ΔHADS depression score (between week 16 and week 0) (p = 0.014) and percentage of depressed patients (p = 0.015). For quality of life, QLQ-C30 global health status score (p = 0.032), Δglobal health status score (between week 16 and week 0) (p = 0.003), and Δfunctional score (between week 16 and week 0) (p = 0.005) were higher in the patient education and rehabilitation program group compared to control group. However, no difference of QLQ-C30 functional score (p = 0.103), QLQ-C30 symptom score (p = 0.808) or Δsymptom score (between week 16 and week 0) (p = 0.680) was observed between two groups.
As urologic oncologists, we are not specifically trained to treat depression and mental health disorders in our bladder cancer patients, however, identifying risk factors and making appropriate consultations to psycho-oncologists is necessary. To further assess this, Mani et al. evaluated the prevalence of mental distress in patients with newly diagnosed bladder cancer, cancer-information internet search behavior, and the influence of information seeking on level of distress. For this study, 101 bladder cancer patients answered the HADS and Fragebogen zur Belastung von Krebskranken (FBK-R23) questionnaires in order to evaluate mental distress and assess questions concerning information seeking. Analysis of mental distress showed that 23.2% had a score above the HADS-A cutoff, 25.3% above the HADS-D cutoff, and 21.4% showed a pathologic FBK-R23 score. Overall, 75% felt well informed about their illness, and active searches for information/ use of the internet did not correlate with the HADS-A, HADS-D, or FBK-R23 score. However, the quality of the urologist's information and the feeling of being informed correlated with the grade of mental distress.
Besides the treatment of bladder cancer, informing patients about their disease in a psychologically wholesome manner and working together with psycho-oncologically trained psychologists are essential tasks for the treating urologist. Furthermore, future studies assessing interventions for improving mental health and outcomes among bladder cancer patients is crucial to identifying impactful interventions and monitoring strategies. Early work suggests that patient education and rehabilitation programs may be helpful in decreasing depression and anxiety among patients with bladder cancer.
Conclusions
Bladder cancer patients are a comorbid population. While often under-appreciated, many patients with bladder cancer have a pre-existing psychiatric diagnosis at the time of cancer diagnosis, and many others will develop mental health disorders after diagnosis. In addition to decreasing quality of life, previous studies have suggested that psychiatric comorbidities can negatively impact cancer-specific and overall survival. Additionally, bladder cancer patients are at a ~70% increased risk of suicidal death compared to the general population/non-cancer patients. While awareness of the importance of mental health in bladder cancer patients is growing, further studies are needed to assess the role of interventions such as cognitive-behavioral therapy or pharmacotherapy in order to optimize treatment.
Published Date: June 2021
- Written by: Zachary Klaassen, MD, MSc, Medical College of Georgia, Augusta, Georgia, USA
- References:
- He H, Xie H, Chen Y, et al. Global, regional, and national burdens of bladder cancer in 2017: estimates from the 2017 global burden of disease study. BMC Public Health 2020; 20(1): 1693.
- Vartolomei L, Ferro M, Mirone V, Shariat SF, Vartolomei MD. Systematic Review: Depression and Anxiety Prevalence in Bladder Cancer Patients. Bladder Cancer 2018; 4(3): 319-26.
- Zhang Y, Wang Y, Song B, Li H. Patients' self-report anxiety, depression and quality of life and their predictive factors in muscle invasive bladder cancer patients receiving adjuvant chemotherapy. Psychol Health Med 2020; 25(2): 190-200.
- Pham H, Torres H, Sharma P. Mental health implications in bladder cancer patients: A review. Urol Oncol 2019; 37(2): 97-107.
- Sathianathen NJ, Fan Y, Jarosek SL, et al. Disparities in Bladder Cancer Treatment and Survival Amongst Elderly Patients with a Pre-existing Mental Illness. Eur Urol Focus 2020; 6(6): 1180-7.
- Klaassen Z, Wallis CJD, Goldberg H, et al. The impact of psychiatric utilisation prior to cancer diagnosis on survival of solid organ malignancies. Br J Cancer 2019; 120(8): 840-7.
- Jazzar U, Yong S, Klaassen Z, et al. Impact of psychiatric illness on decreased survival in elderly patients with bladder cancer in the United States. Cancer 2018; 124(15): 3127-35.
- Jazzar U, Bergerot CD, Shan Y, et al. Use of psychotropic drugs among older patients with bladder cancer in the United States. Psychooncology 2021; 30(6): 832-43.
- Raphael MJ, Griffiths R, Peng Y, et al. Mental Health Resource Use Among Patients Undergoing Curative Intent Treatment for Bladder Cancer. J Natl Cancer Inst 2021.
- Misono S, Weiss NS, Fann JR, Redman M, Yueh B. Incidence of suicide in persons with cancer. J Clin Oncol 2008; 26(29): 4731-8.
- Klaassen Z, Jen RP, DiBianco JM, et al. Factors associated with suicide in patients with genitourinary malignancies. Cancer 2015; 121(11): 1864-72.
- Klaassen Z, Goldberg H, Chandrasekar T, et al. Changing Trends for Suicidal Death in Patients With Bladder Cancer: A 40+ Year Population-level Analysis. Clin Genitourin Cancer 2018; 16(3): 206-12 e1.
- Guo Z, Gu C, Li S, et al. Incidence and risk factors of suicide among patients diagnosed with bladder cancer: A systematic review and meta-analysis. Urol Oncol 2021; 39(3): 171-9.
- Klaassen Z, Wallis CJD, Chandrasekar T, et al. Cancer diagnosis and risk of suicide after accounting for prediagnosis psychiatric care: A matched-cohort study of patients with incident solid-organ malignancies. Cancer 2019; 125(16): 2886-95.
- Klaassen Z, Lokeshwar SD, Lowery-Allison A, Wallis CJD. Mental Illness and Bladder Cancer Patients: The Time for Assertive Intervention Is Now. Eur Urol Focus 2020; 6(6): 1188-9.
- Bessa A, Rammant E, Enting D, et al. The need for supportive mental wellbeing interventions in bladder cancer patients: A systematic review of the literature. PLoS One 2021; 16(1): e0243136.
- Li Z, Wei D, Zhu C, Zhang Q. Effect of a patient education and rehabilitation program on anxiety, depression and quality of life in muscle invasive bladder cancer patients treated with adjuvant chemotherapy. Medicine (Baltimore) 2019; 98(44): e17437.
Implications of Guideline-Based, Risk-Stratified Restaging Transurethral Resection of High-Grade Ta Urothelial Carcinoma on Bacillus Calmette-Guérin Therapy Outcomes - Beyond the Abstract
Institute for Clinical and Economic Review: BCG Unresponsive Disease
The ICER Process
To address the importance of high-value care in the context of affordability and access, the Institute for Clinical and Economic Review (ICER) an organization whose mission is to conduct evidence-based reviews of health care interventions, independently reviews evidence, free from financial conflicts of interest, to understand an intervention’s ability to extend or improve life, a fair price based on clinical evidence, and how stakeholders can translate evidence into real-world insurance coverage to improve patient outcomes. The ICER process is multi-fold, rigorous, inclusive and systematic, based upon a Value Assessment Framework conducted in 5 steps.1
- Written by: Yair Lotan, Jonathan L Wright, and Angela B Smith
- References:
1. https://icer.org/our-approach/methods-process/
2. Girish S Kulkarni 1, Antonio Finelli, Neil E Fleshner, Michael A S Jewett, Steven R Lopushinsky, Shabbir M H Alibhai. Optimal management of high-risk T1G3 bladder cancer: a decision analysis. PLoS Med. 2007 Sep;4(9):e284.
3. Lerner SP, Bajorin DF, Dinney CP, Efstathiou JA, Groshen S, Hahn NM, Hansel D, Kwiatkowski D, O'Donnell M, Rosenberg J, Svatek R, Abrams JS, Al-Ahmadie H, Apolo AB, Bellmunt J, Callahan M, Cha EK, Drake C, Jarow J, Kamat A, Kim W, Knowles M, Mann B, Marchionni L, McConkey D, McShane L, Ramirez N, Sharabi A, Sharpe AH, Solit D, Tangen CM, Amiri AT, Van Allen E, West PJ, Witjes JA, Quale DZ. Summary and Recommendations from the National Cancer Institute's Clinical Trials Planning Meeting on Novel Therapeutics for Non-Muscle Invasive Bladder Cancer.. Bladder Cancer. 2016 Apr 27;2(2):165-202. doi: 10.3233/BLC-160053.PMID: 27376138
4. Svatek RS, Hollenbeck BK, Holmäng S, Lee R, Kim SP, Stenzl A, Lotan Y. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. 2014 Aug;66(2):253-62. doi: 10.1016/j.eururo.2014.01.006. Epub 2014 Jan 21.PMID: 24472711
5. Hu JC, Chughtai B, O'Malley P, Halpern JA, Mao J, Scherr DS, Hershman DL, Wright JD, Sedrakyan A. Perioperative Outcomes, Health Care Costs, and Survival After Robotic-assisted Versus Open Radical Cystectomy: A National Comparative Effectiveness Study.. Eur Urol. 2016 Jul;70(1):195-202. doi: 10.1016/j.eururo.2016.03.028. Epub 2016 Apr 28.PMID: 27133087


