(UroToday.com) The 2024 Advanced Prostate Cancer Consensus Conference (APCCC) held in Lugano, Switzerland was host to a session addressing the management of metastatic hormone-sensitive prostate cancer (mHSPC). Dr. Thomas Zilli discussed which patients with metachronous low-volume mHSPC should be recommended for ‘total therapy’ with metastasis-directed therapy (MDT).
Dr. Zilli noted that there remain three main open questions for ‘total therapy’ with MDT:
- What is the evidence to support MDT in this setting?
- Should we intensify MDT with concurrent systemic therapy?
- How should we treat our patients with MDT in 2024 and beyond?
In 2017, the ‘Pokemet’ approach to metastatic prostate cancer was coined, with investigators hypothesizing whether we should target and treat all visible sites of metastatic disease with the main goal being to avoid the potential toxicity from androgen suppression.
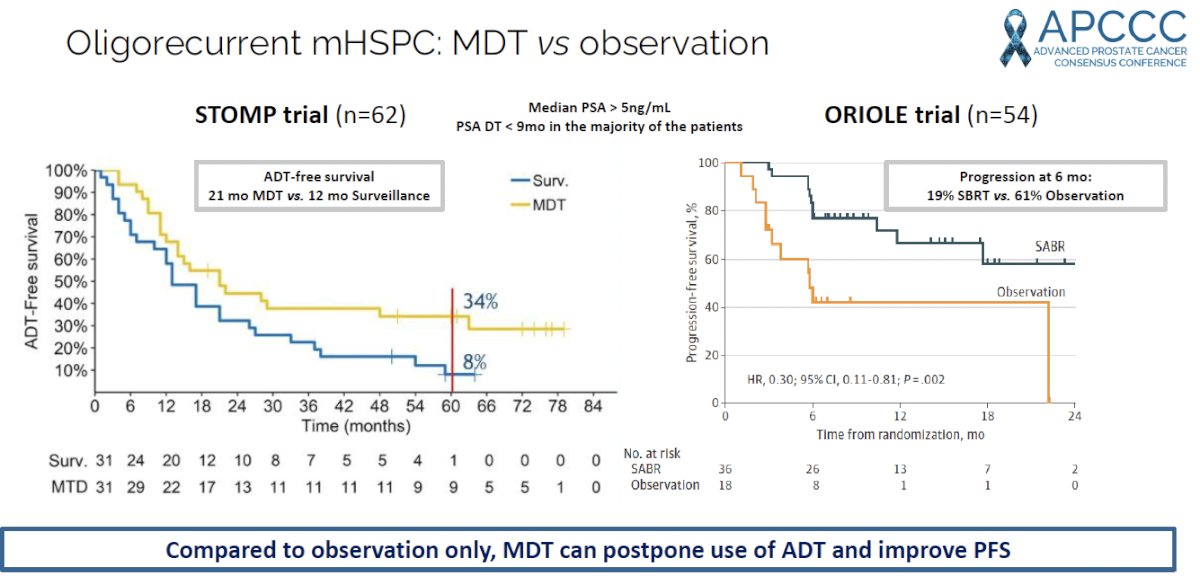
Evidence to inform this decision comes from two prominent phase II trials in the oligorecurrent, metastatic hormone sensitive prostate cancer (mHSPC): STOMP and ORIOLE.1,2
The STOMP trial was a multicenter, randomized phase II trial that prospectively evaluated the effects of MDT for patients with evidence of oligometastatic disease on choline PET/CT (up to three extracranial sites) who had received prior treatment with curative intent and had evidence of biochemical recurrence with testosterone >50 ng/ml (i.e. metachronous, oligometastatic mHSPC). Between 2012 and 2015, 62 patients were randomized 1:1 and MDT was either SBRT or metastasectomy. The primary endpoint was time to initiation of ADT (called ADT-free survival). ADT was initiated for symptoms, progression beyond three metastases, or local progression of known metastatic disease. Time to castration resistance was a secondary endpoint (called CRPC-free survival). With a median follow up of 5.3 years, the five-year ADT-free survival was 8% in the surveillance arm compared to 34% for the MDT group (HR: 0.57, 95% CI: 0.38-0.84, log-rank p=0.06). No differences were seen between groups when stratified by nodal versus non-nodal metastases. The secondary endpoint of CRPC-free survival at 5 years was 53% in subjects under surveillance and 76% in those receiving MDT (HR: 0.62, 80% CI: 0.35-1.09).
The ORIOLE trial was a randomized phase II trial of 54 men with metachronous, oligometastatic mHSPC (up to three sites). Metastatic sites were diagnosed via conventional imaging (CT, MRI, and/or radionuclide bone scan). Between 2016 and 2018, patients were randomized in a 2:1 fashion to receive SABR or observation. The primary outcome was progression at 6 months, defined as serum PSA increase, progression detected by conventional imaging, symptomatic progression, ADT initiation for any reason, or death. Progression at six months occurred in 7 of 36 patients (19%) receiving SABR and 11 of 18 patients (61%) undergoing observation (p=0.005). Treatment with SABR improved median PFS (not reached versus 5.8 months; HR: 0.30; 95% CI: 0.11 – 0.81; p=0.002). No grade ≥3 toxic effects were observed.
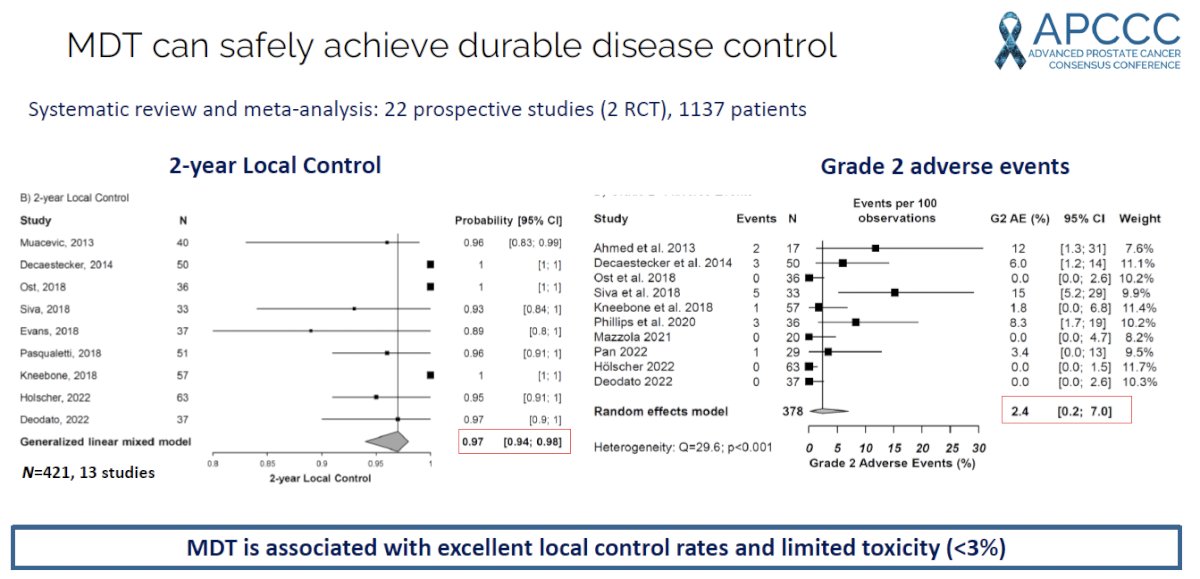
A systematic review and meta-analysis of 22 prospective studies of MDT in patients with metastatic prostate cancer demonstrated that the estimated 2-year progression-free survival was 46%, with 2-year local control, ADT-free survival, and overall survivals of 97%, 55%, and 97% respectively. It appears MDT is associated with a favorable safety profile with grade 2 and grade ≥3 adverse events occurring in 2.4% and 0.3% of patients, respectively.3
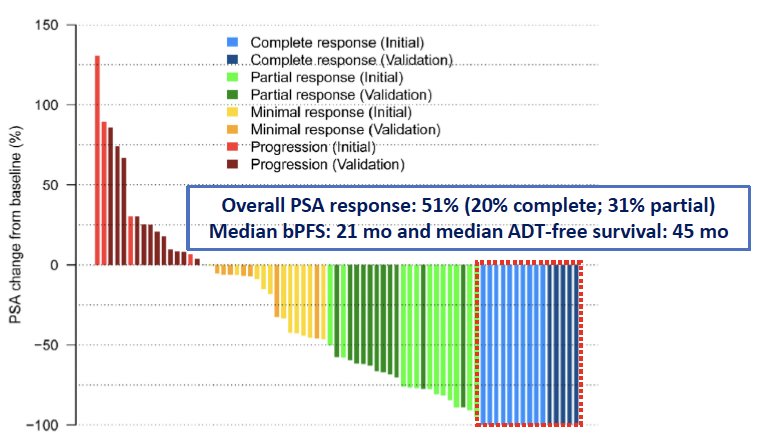
In addition to delaying the time to ADT, it appears that MDT alone may have the potential to cure select patients with oligometastatic disease. In 2022, Glicksman et al. published the results of two sequential single-arm phase II trials of 74 patients with biochemical recurrence (PSA: 0.4–3 ng/mL) and negative conventional imaging following radical prostatectomy and postoperative radiation therapy. All patients underwent 18F-DCFPyL positron emission tomography/computed tomography (PET/CT). Patients with molecularly defined oligorecurrent prostate cancer underwent MDT with SBRT (n=64) or surgery (n=10), without concurrent ADT. The primary endpoint was biochemical response (≥50% PSA decline from baseline). Among the 74 patients, the median number of PSMA-PET/CT positive lesions was two, with 87% of recurrences nodal in location. At a median follow-up of 24 months, the biochemical response rate was 51% (20% complete and 31% partial). The median biochemical progression-free survival was 21 months, and the median ADT-free survival was 45 months. Based on these promising results, Dr. Zilli noted that complete biochemical responses can be achieved with MDT alone in select patients PSMA-defined oligorecurrent prostate cancer.
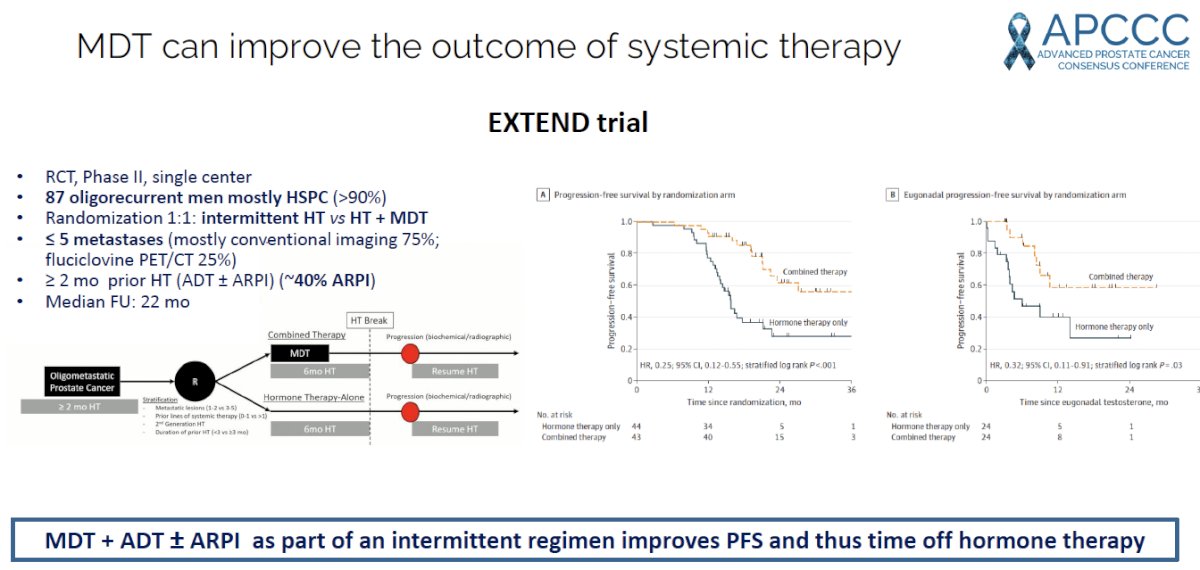
More recently, this “Pokemet’ approach appears to be evolving with MDT being evaluated in combination with, not in lieu of, systemic therapy. The EXTEND trial was a single center, phase II randomized controlled trial of 87 oligorecurrent men, mostly with mHSPC (>90%), who were randomized 1:1 to intermittent hormone therapy +/- MDT (definitive radiation therapy to all sites of disease). All patients had ≤5 metastases, as defined by conventional imaging (75%) or fluciclovine PET/CT (25%). A planned break in hormone therapy occurred 6 months after enrollment, after which hormone therapy was withheld until progression. At a median follow-up of 22 months, progression-free survival was improved in the combined therapy arm (HR: 0.25, 95% CI: 0.12 – 0.55, p<0.001). Significantly, ‘eugonadal’ progression-free survival was also improved with this combination approach (HR: 0.32, p=0.03).4
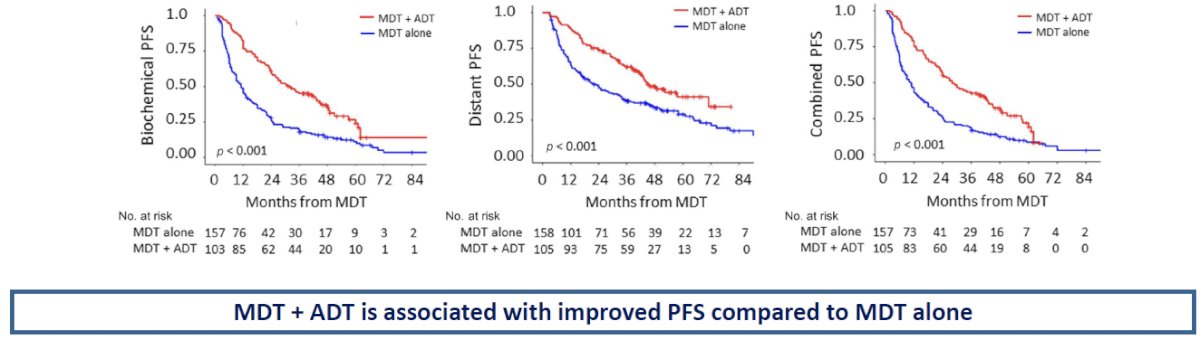
The converse appears to be true as well (i.e., the addition of ADT to MDT is superior to MDT alone). A recent multi-institutional, retrospective analysis of 263 patients with conventional imaging-defined oligometastatic mHSPC demonstrated that the use of MDT + ADT, versus MDT alone, was associated with superior:5
- Biochemical progression-free survival: 25% versus 11%
- Distant progression-free survival: 41% versus 29%
- Combined biochemical or distant progression-free survival: 19% versus 9%
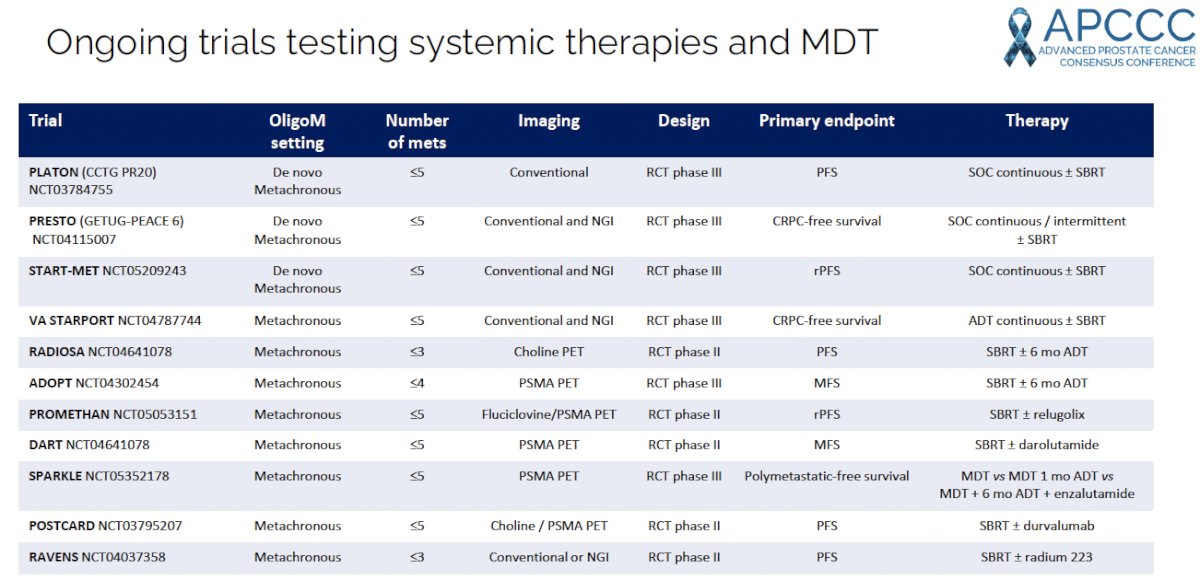
There are currently numerous ongoing trials testing the combination of systemic therapy + MDT.
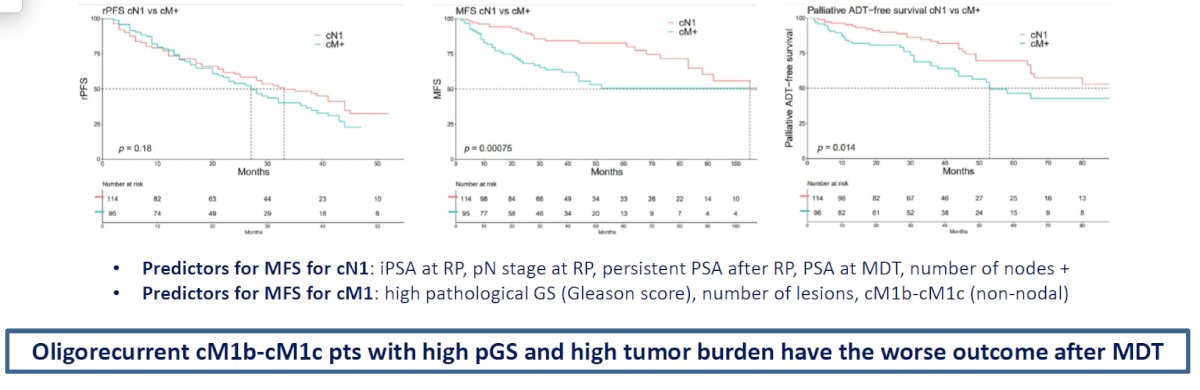
Can we predict which patients will relapse with MDT? In 2023 Milenkovic et al. published the results of a bicentric, retrospective study, which included consecutive patients who underwent MDT for oligorecurrent prostate cancer following radical prostatectomy, performed between 2006 and 2020.6 MDT included the following modalities:
- SBRT (23%)
- Salvage lymph node dissection (56%)
- Whole pelvis/retroperitoneal radiation therapy (15%)
- Metastasectomy (5%)
Overall, the investigators demonstrated that oligorecurrent cM1b-c patients with higher pathologic Gleason Score and high tumor burden have the worst outcomes after MDT.
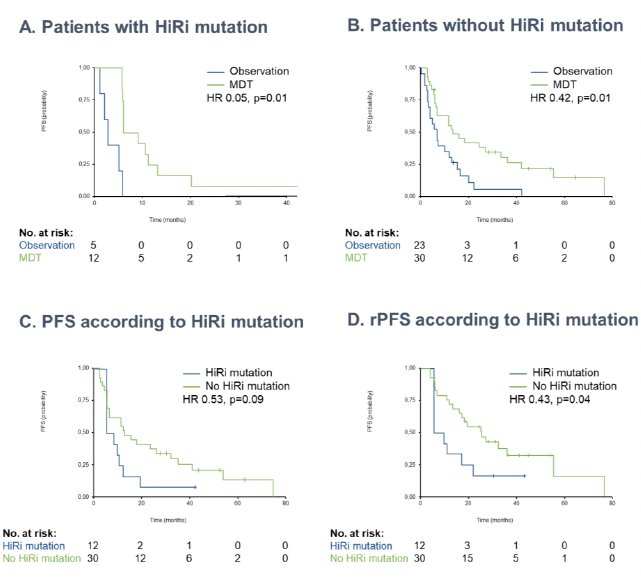
In addition to clinicopathologic variables, it appears that genomic profiling may provide prognostic value in the MDT setting. A pooled analysis of 70 prostate cancer patients with available genomic data from STOMP and ORIOLE demonstrated that patients with high-risk mutations (ATM, BRCA1/2, RB1, or TP53) derived the largest progression-free survival benefit from MDT (HR for high-risk=0.05; HR for no high-risk=0.42; p-value for interaction=0.12). Within the MDT cohort, the progression-free survival was 13.4 months in those without a high-risk mutation, compared with 7.5 months in those with a high-risk mutation (HR: 0.53; 95% CI: 0.25 – 1.11; p=0.09).7
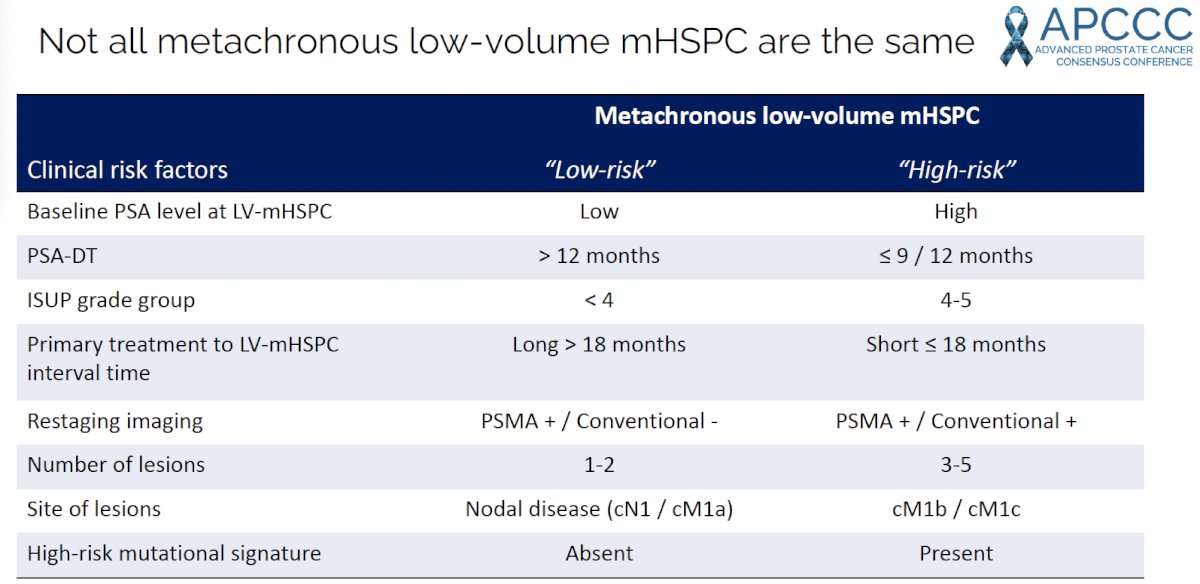
Overall, when considering MDT for patients with oligorecurrent or low-volume metachronous mHSPC, it is important to risk-stratify these patients based on the following clinical risk factors:
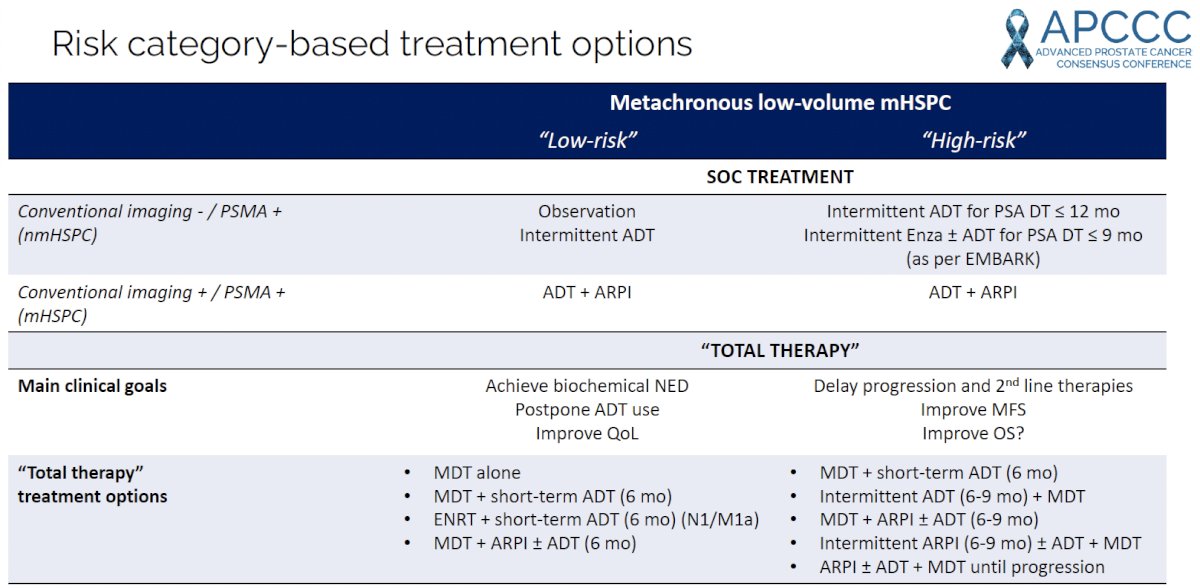
Dr. Zilli proposed the following risk category-based treatment algorithm, summarized in the table below, based on clinical risk (low versus high-risk) and conventional imaging/PSMA findings.
Dr. Zilli concluded his presentation with the following take-home messages:
- MDT is a rapidly evolving field, even if it is not yet considered standard of care for metachronous low-volume mHSPC
- MDT provides excellent disease control with minimal toxicity
- In combination with systemic therapies, MDT can improve outcomes compared to systemic therapy alone, but there remain open questions regarding the optimal duration and type
- Have multidisciplinary discussions and enroll in trials or prospective cohorts
Presented by: Thomas Zilli, MD, Professor, Radiation Oncology Department, Geneva University Hospital, Geneva, Switzerland
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 Advanced Prostate Cancer Consensus Conference (APCCC) Meeting, Lugano, Switzerland, Thurs, Apr 25 - Sat, Apr 27, 2024.
References:
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer -The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-659.
- Miszczyk M, Rajwa P, Yanagisawa T, et al. The Efficacy and Safety of Metastasis-directed Therapy in Patients with Prostate Cancer: A Systematic Review and Meta-analysis of Prospective Studies. Eur Urol. 2024;2:125-138.
- Tang C, Sherry AD, Haymaker C, et al. Addition of Metastasis-Directed Therapy to Intermittent Hormone Therapy for Oligometastatic Prostate Cancer: The EXTEND Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023;9(6): 825-834.
- Deek MP, Sutera P, Jing Y, et al. Multi-institutional Analysis of Metastasis-directed Therapy with or Without Androgen Deprivation Therapy in Oligometastatic Castration-sensitive Prostate Cancer. Eur Urol Oncol. 2024: S2588-9311(24)00086-5.
- Milenkovic U, Kuijk J, Roussel E, et al. Predictors of Recurrence After Metastasis-directed Therapy in Oligorecurrent Prostate Cancer Following Radical Prostatectomy. Eur Urol Oncol. 2023;6(6): 582-589.
- Deek MP, van der Eecken K, Sutera P, et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022;40(29): 3377-3382.
Related Content: