Figure 1 – Timeline of FDA approvals in urothelial cancer:
To assess whether the treatment is working we need excellent biomarkers. There are several different classes of biomarkers that we need to recognize:
- Diagnostic - which identify the presence of a malignancy
- Prognostic - which demonstrate the baseline characteristic associated with survival
- Predictive - which identifies patients most likely to benefit from a specific intervention
- Pharmacodynamic - which monitor tumor response to a specific intervention
- Surrogate - which serve as an intermediate endpoint for survival
The current known and used biomarkers in metastatic urothelial carcinoma are shown in Table 1.
Table 1 – Biomarkers in metastatic urothelial cancer:
The IMvigor130 Trial (Figure 2) will randomize locally advanced or metastatic urothelial carcinoma patients with no prior systemic therapy to three arms:
- Arm A – Atezolizumab + plt/gem therapy
- Arm B – Atezolizumab monotherapy
- Arm C – Placebo + plt/gem therapy
The primary endpoints in this trial were progression-free survival and overall survival. Additionally, there were other exploratory biomarker analyses. The intention to treat population consisted of 1213 patients and several biomarkers were evaluated. Figure 3 demonstrates the primary progression-free survival and interim overall survival analysis, demonstrating a clear benefit associated with arm A that included patients treated with atezolizumab and plt/gem.
Figure 2 – Phase 3 IMvigor trial design:
Figure 3 – Primary progression-free survival and interim overall survival analysis of the IMvigor130 study:
One of the markers that was analyzed was F-TBRS signature. The analysis shows that patients with high level of this biomarker had improved overall survival (Figure 4), when treated with atezolizumab + plt/gem, and placebo + plt/gem, but interestingly, not with atezolizumab monotherapy.
However, other biomarker defined populations may derive particular benefit from treatment regiments containing atezolizumab.
Figure 4 – High overall survival in patients with F-TBRS signature: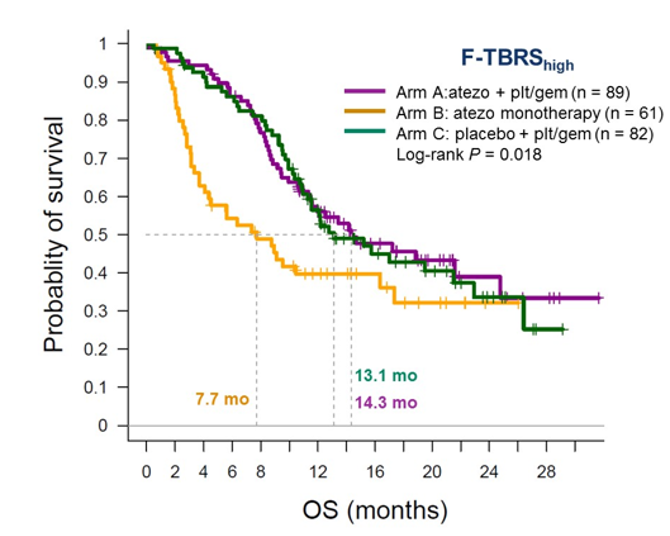
Dr. Lang moved on to discuss the setting of neoadjuvant treatment in urothelial carcinoma. In contrast to the metastatic urothelial cancer, there has been little change in the neoadjuvant settin since 2005, when MVAC neoadjuvant chemotherapy was introduced. The DUTRENEO study, presented in ASCO 2020 virtual meeting attempted to assess the role of immune checkpoint inhibitors in the neoadjuvant setting (Figure 5). The primary objective of this study was to measure anti-tumor activity manifested as pathological complete response rate. Secondary objectives included disease-free survival, overall survival and toxicity profile; and the exploratory objective was to assess the biomarkers of response in tumor samples.
Figure 5 – DUTRENEO study design:
The responses in the intention to treat population are shown in Table 2, the PDL-1 expression in tumor and immune cells by TIS is shown in Figure 6, and lastly, the pathological complete response rate by histology is shown in Figure 7.
Table 2 – Responses in the intention to treat populations in the DUTRENEO trial: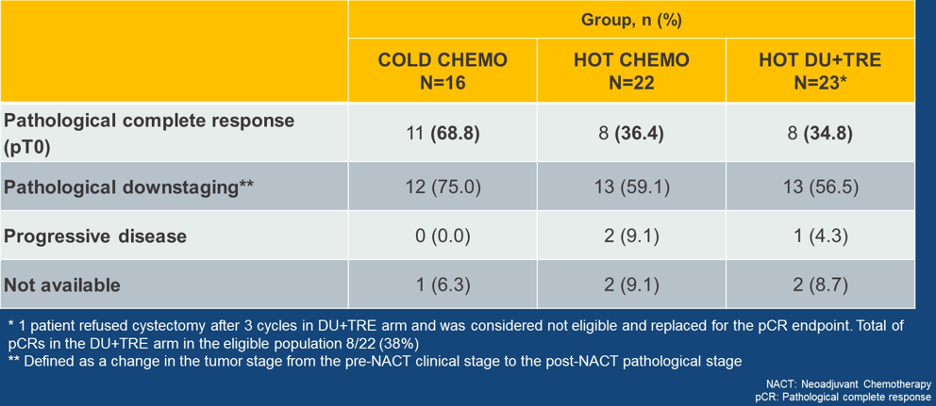
Figure 6 – PD-L1 expression in tumor and immune cells by TIS in the DUTRENEO trial:
Figure 7 – Pathological complete response rate by histology in the DUTRENEO trial: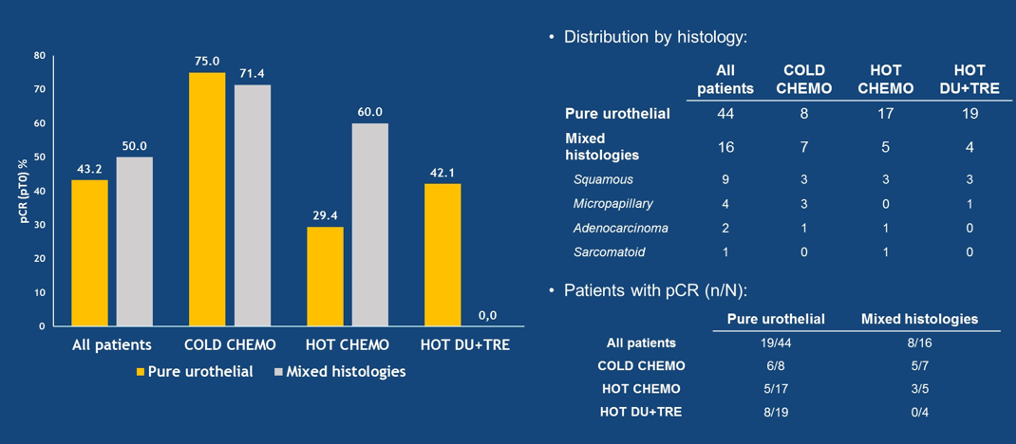
Dr. Lang concluded his talk with some important discussion points. The new therapeutic advances in urothelial carcinoma drive us to ask how we can identify those patients that are most likely to benefit from novel treatment and those that don’t. The IMvigor 130 study presented a well-defined population with full clinical annotation and survival outcomes. The initial results identified promising biomarkers of resistance to atezolizumab monotherapy. It is not clear if these novel biomarkers are ready to go through the regulatory process for FDA clearance. It is also not clear if new combination strategies can be developed based on these interesting results.
Lastly, the DUTRENEO trial is among the first trials in urothelial carcinoma to randomize patients based on biomarker results in the neoadjuvant setting. This trial design is necessary to define a predictive biomarker and while the study results are negative, this trial has shown that this design is feasible in urothelial carcinoma. Therefore, this trial design should be considered for other trials and biomarkers in the neoadjuvant space.
Presented by: Joshua M. Lang, MD, Assistant Professor in the Department of Medicine at the University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, Carbone Cancer Center.
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, NY, USA, @GoldbergHanan at the 2020 American Society of Clinical Oncology Virtual Annual Meeting (#ASCO20), May 29th-May 31st, 2020
Related Content:
Read: ASCO 2020: Tumor Microenvironment Biomarkers Associated with Overall Survival from the Phase III IMvigor130 Study in Locally Advanced or Metastatic Urothelial Cancer
Read: ASCO 2020: DUTRENEO Trial: A Randomized Phase II Trial of DUrvalumab and TREmelimumab versus Chemotherapy as a NEOadjuvant Approach to Muscle-Invasive Urothelial Bladder Cancer Patients


