Table 1. Studies assessing the role of prostate MRI in local staging of prostate cancer

In Abstract 5513 presented by Dr. David Eldred-Evans et al. at the 2020 American Society of Clinical Oncology Virtual Annual Meeting, the authors from the Imperial College in London performed a paired screen positive study design in two screening centers. Men aged 50 to 69 years were invited for screening from seven primary care practices and using community-based recruitment. The men underwent screening with both a prostate-specific antigen (PSA) blood test, with a result of above 3 ng/ml considered positive, and a multiparametric MRI of the prostate (either 1.5 or 3 T with PIRADS 2.0 threshold ≥ 3 or ≥ 4 considered positive). All participants were blinded to the indication from biopsy, and all reporters were blinded to the results of the other tests. If both screening tests (PSA and MRI) were positive, the patients were referred for transperineal systemic 12 core biopsy +/- targeted biopsy. The clinically significant disease was sought out, defined as any Gleason score ≥ 3+4 (Figure 1).
Figure 1. Abstract 5513, ASCO 2020 study design
The authors also noted much faster recruitment rates than anticipated initially (Figure 2).
Figure 2. Recruitment rate in Abstract 5513
The results of this study, shown in Table 2, demonstrated considerably higher sensitivity and specificity of multiparametric MRI using a PIRADS 2.0 score of ≥ 4 when compared to PSA ≥ 3 ng/ml.
Table 2. Abstract 5513 results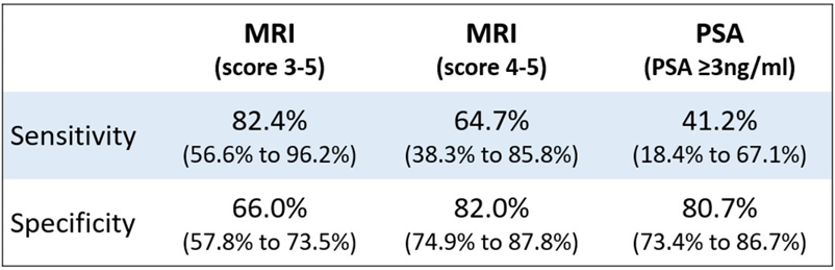
The authors of the study concluded that these results support a large-scale evaluation of the clinical impact and cost-effectiveness of using a short MRI scan, with PIRADS 4 to 5 score as a cut-off to denote a screen-positive test.
The next abstract discussed was Abstract 5512 entitled: “Late toxicities and recurrences in patients with clinical stage one nonseminomatous germ cell tumor after one cycle of adjuvant BEP vs. primary retroperitoneal lymph node dissection – A 13 years follow-up analysis of a phase III trial cohort”.
This was a follow-up study to an original study that was published back in 2008 in the Journal of Clinical Oncology, a randomized Phase III trial comparing retroperitoneal lymph node dissection (RPLND) with one course of BEP chemotherapy in the adjuvant treatment of clinical stage one non-seminoma testicular germ cell tumor.1 This was a multicenter study including 61 centers in Germany taking place between 1996 and 2005. A total of 382 patients were randomized to receive either retroperitoneal lymph node dissection or one cycle of BEP chemotherapy. After a median follow-up of 4.7 years, there were only two vs. 15 recurrences in favor of BEP. The hazard ratio to experience tumor recurrences after RPLND vs. chemotherapy was 7.9 (Figure 3).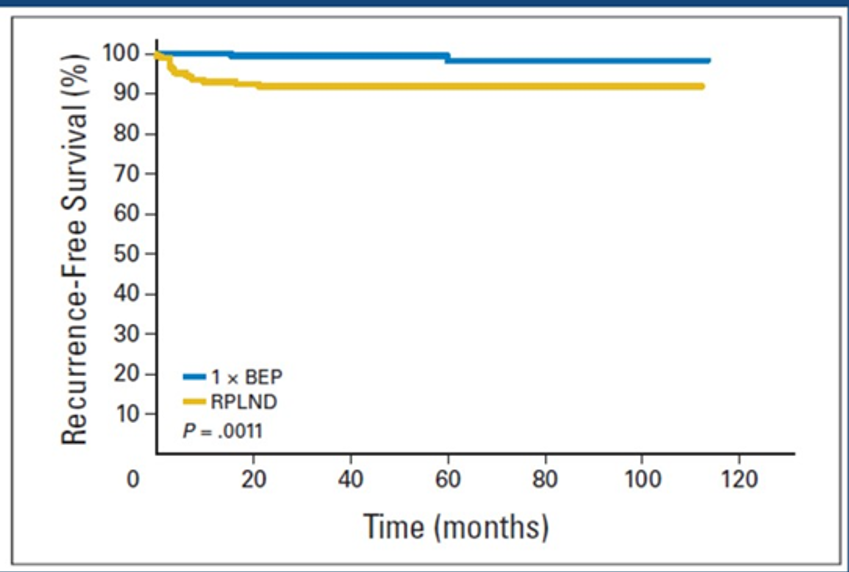
Figure 3. Comparison of BEP chemotherapy to retroperitoneal lymph node dissection in the German multicenter randomized trial (1996-2005)
The follow-up results of this study, presented at the ASCO 2020 Virtual Annual Meeting, demonstrated an additional single recurrence in both arms (RPLND and BEP), as seen in Table 3. Retrograde ejaculation was significantly more common in the RPLND arm (24% vs. 9%, p=0.01).
Table 3. Follow-up and outcome in Abstract 5512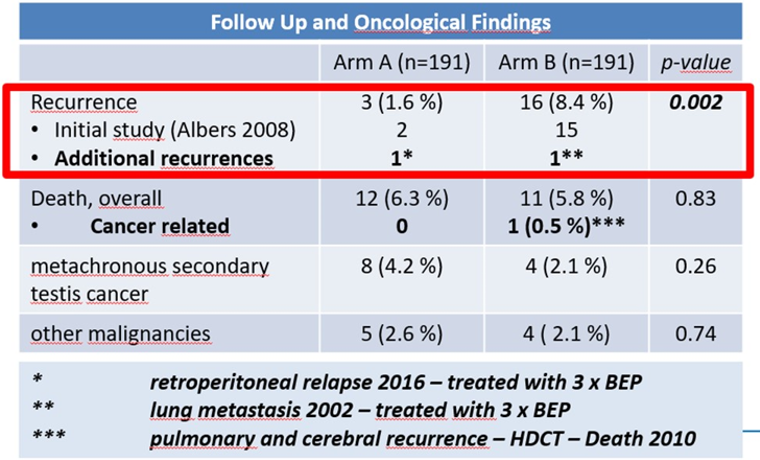
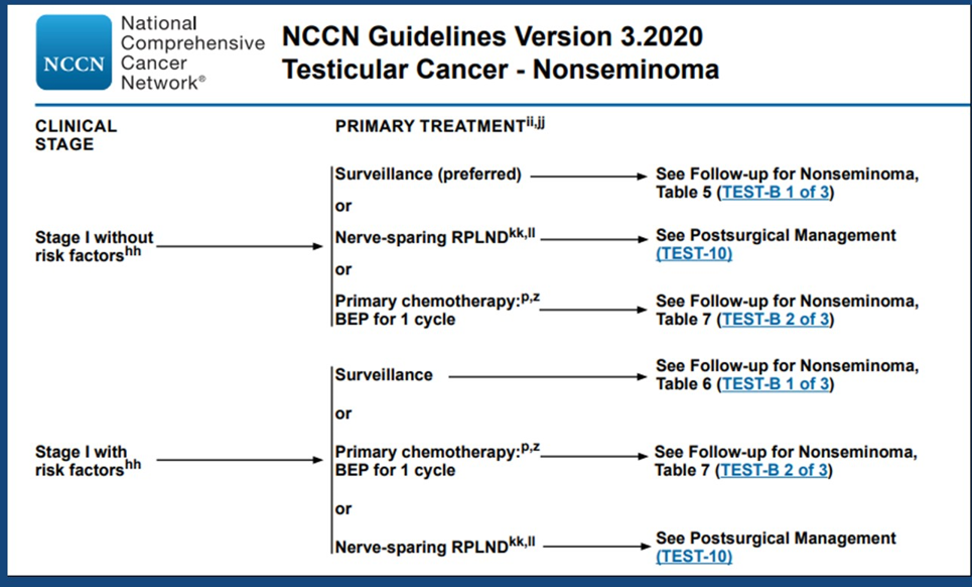
Table 4. NCCN guidelines
Presented by: Timur Mitin, MD, PhD, Medical Director, Tuality OHSU Cancer Center, Associate Professor of Radiation Medicine, Oregon Health and Science University, Portland, Oregon
Written by: Hanan Goldberg, MD, MSc., Urology Department, SUNY Upstate Medical University, Syracuse, New York, Twitter: @GoldbergHanan, at the 2020 American Society of Clinical Oncology Virtual Annual Meeting (#ASCO20), May 29th-May 31st, 2020
References:
- Albers, Peter, Roswitha Siener, Susanne Krege, Hans-Uwe Schmelz, Klaus-Peter Dieckmann, Axel Heidenreich, Peter Kwasny, et al. "Randomized phase III trial comparing retroperitoneal lymph node dissection with one course of bleomycin and etoposide plus cisplatin chemotherapy in the adjuvant treatment of clinical stage I nonseminomatous testicular germ cell tumors: AUO trial AH 01/94 by the German Testicular Cancer Study Group." Journal of Clinical Oncology 26, no. 18 (2008): 2966-2972.
- Honecker, F., J. Aparicio, D. Berney, Jörg Beyer, C. Bokemeyer, R. Cathomas, N. Clarke et al. "ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up." Annals of oncology 29, no. 8 (2018): 1658-1686.
- Cohn‐Cedermark, G., O. Stahl, T. Tandstad, and Swenoteca. "Surveillance vs. adjuvant therapy of clinical stage I testicular tumors–a review and the SWENOTECA experience." Andrology 3, no. 1 (2015): 102-110.


