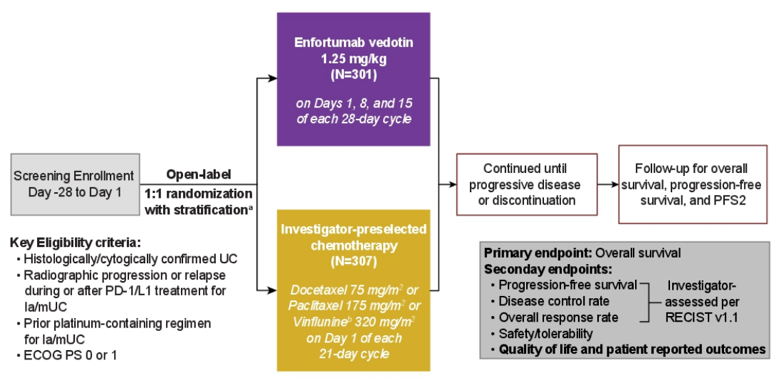
The study design for EV-301 is as follows:
Patients completed the validated European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire Core 30 (QLQ-C30) at baseline, on Day 1 of each week for the first 12 weeks, and then every 12 weeks until discontinuation. The QLQ-C30 assessed functional domains, symptom scales/items, financial impact, and overall health/QoL. Descriptive statistics were used to summarize instrument compliance rates and scores, and mixed model repeated measures were used to evaluate changes from baseline over time. Logistic regressions were conducted to assess confirmed improvement rates, defined as clinically meaningful improvement (predefined per domain) over two subsequent visits.
Of the 608 randomized patients (enfortumab vedotin, n = 301; standard chemotherapy, n = 307), 77.3% were male, median age was 68 (range: 30-88), and 30.9% had liver metastasis.The questionnaire completion rate was 60% for enfortumab vedotin and 43% for chemotherapy on Day 8, with rates falling to 44% and 34% at week 12, respectively. Questionnaire compliance rates at baseline were ̃90% in both groups: during the study, average rates were 70.2% (enfortumab vedotin) and 66.9% (standard chemotherapy). Baseline QLQ-C30 scores were similar between groups. At Week 12, scores on the global health status scale were similar between groups (enfortumab vedotin: -2.8, standard chemotherapy: -5.0; p = 0.2429), but standard chemotherapy was associated with numerically greater deterioration and more variability in quality of life over the first 12 weeks.
Patients receiving enfortumab vedotin had significant reduction in pain symptoms (enfortumab vedotin: -5.62, standard chemotherapy: +0.11; adjusted difference: -5.73, p < 0.05), but significant worsening of appetite loss (enfortumab vedotin: +8.55, standard chemotherapy: +1.26; adjusted difference: 7.29, p < 0.05) compared with standard of chemotherapy. Other symptom scores were not significantly different between groups.
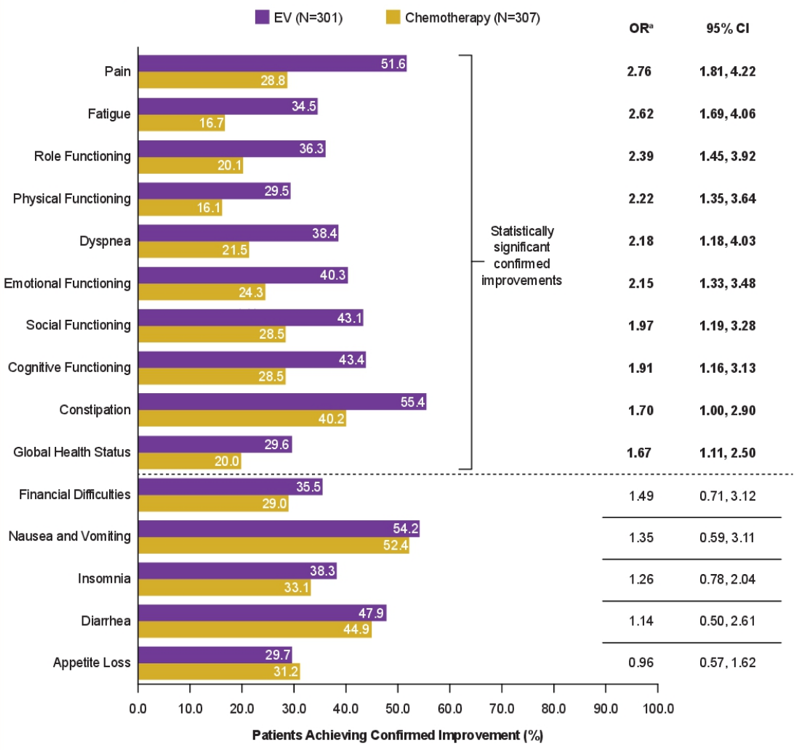
Higher proportions of patients on enfortumab vedotin vs standard chemotherapy had significant confirmed improvements across all functioning domains (role, physical, emotional, social, cognitive), global heath status, and several symptom scales (pain, fatigue, dyspnea, constipation). Notably, the greatest difference in improvement was reported for pain (enfortumab vedotin: 51.6%, standard chemotherapy: 28.8%; OR 2.76, 95% CI 1.81, 4.22):
Dr. Mamtani concluded his quality of life presentation for the EV-301 trial with the following conclusions:
- Quality of life was maintained cross the course of study treatment with patients receiving enfortumab vedotin
- Enfortumab vedotin treated patients had statistically significant reductions in pain symptoms compared with chemotherapy-treated patients, however enfortumab vedotin treated patients had significantly more appetite loss
- Significantly more patients had confirmed improvement in the majority of domains, with clinically meaningful improvements 1.6 to 2.7 times higher across all functioning and most symptom scores
Clinical trial information: NCT03474107
Presented by: Ronac Mamtani, MD, MSCE Assistant Professor of Medicine at the Hospital of the University of Pennsylvania
Co-Authors: Jonathan E. Rosenberg, Thomas Powles, Guru P. Sonpavde, Yohann Loriot, Ignacio Duran, Jae-Lyun Lee, Nobuaki Matsubara, Christof Vulsteke, Daniel Castellano, Srikala S. Sridhar, Helle Pappot, Begoña P. Valderrama, Howard Gurney, Jens Bedke, Michiel Simon Van Der Heijden, Chunzhang Wu, Zsolt Hepp, Caroline McKay, Daniel P. Petrylak; Genitourinary Medical Oncology Service, Division of Solid Tumor Oncology, Memorial Sloan Kettering Cancer Center, New York, NY; Barts Cancer Institute, Cancer Research UK Experimental Cancer Medicine Centre, Queen Mary University of London, Royal Free National Health Service Trust,, London, United Kingdom; Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA; Department of Cancer Medicine, Gustave Roussy, Université Paris-Saclay, Villejuif, France; Hospital Universitario Marqués de Valdecilla, IDIVAL, Cantabria, Spain; Asan Medical Center and University of Ulsan College of Medicine, Seoul, South Korea; Division of Breast and Medical Oncology, National Cancer Center Hospital East, Chiba, Japan; Center for Oncological Research (CORE), University of Antwerp, Integrated Cancer Center Ghent, Ghent, Belgium; Hospital Universitario 12 de Octubre, Madrid, Spain; Cancer Clinical Research Unit, Princess Margaret Cancer Centre, Toronto, ON, Canada; Rigshospitalet, University Hospital of Copenhagen, Copenhagen, Denmark; Department of Medical Oncology, Hospital Universitario Virgen del Rocío, Seville, Spain; Macquarie University Hospital, Sydney, NSW, Australia; Department of Urology, University of Tubingen, Tuebingen, Germany; Netherlands Cancer Institute, Amsterdam, Netherlands; Astellas Pharma, Inc., Northbrook, IL; Seagen Inc., Bothell, WA; Astellas Pharma Inc, Northbrook, IL; Yale School of Medicine, New Haven, CT
Written by: Zachary Klaassen, MD, MSc, Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021
Related Content:
ASCO GU 2021: Primary results of EV-301: A Phase III Trial of Enfortumab Vedotin vs Chemotherapy in Patients With Previously Treated Locally Advanced or Metastatic Urothelial Carcinoma


