She began by emphasizing that the natural history of prostate cancer, even in its metastatic state, is measured in years. Additionally, the many disease states in this progression of the disease have numerous treatment options, with an increasing number recently.
Dr. Horvath began by discussing the results of the PEACE-1 trial, as presented by Dr. Karim Fizazi. She emphasized that this trial included men with de novo metastatic castration sensitive prostate cancer (mCSPC), assessing whether the addition of abiraterone to the standard of care improves outcomes for these patients. Importantly, the standard of care evolved over the course of the study accrual, with initial patients receiving ADT alone and then docetaxel becoming a mandated approach.
While overall survival data are immature, the data presented herein demonstrated a benefit of radiographic progression-free survival of 2.5 years, with a consistent benefit whether the standard of care included docetaxel or not.
Dr. Horvath highlighted that while there is a fairly well-accepted standard of care for patients with high volume mHSPC (involving treatment intensification with docetaxel or an androgen receptor signaling inhibitor), this is somewhat less clear for patients with low-volume disease. At the 2019 APCCC, only 20% of delegates supported the inclusion of docetaxel for men with mCSPC. Thus, the applicability of the standard of care used in PEACE-1 is somewhat debatable.
She further emphasized that while PEACE-1 is strengthened as the largest study to address the question of triplet therapy and has met one of its two co-primary endpoints, there are notable limitations. Most notably, thus far, radiographic progression-free survival is the only reported endpoint. This has not been validated as a surrogate for overall survival so the clinical importance of these findings are somewhat questionable. Relatedly, longer follow-up is required to allow for maturation of overall survival data. Additionally, PEACE-1 highlights the importance of evolving and adapting study design with changes in the standard of care.
Dr. Horvath emphasized that ENZAMET is the closest comparison given that it provided stratified results by docetaxel use. Similar to PEACE-1, ENZAMET demonstrated an improvement in radiographic progression-free survival for patients who received concurrent enzalutamide and docetaxel. However, this did not translate to an overall survival benefit in analyses reported to date. Thus, she cautioned enthusiasm for this triplet approach until it shows evidence of survival benefit.
She then moved to discuss abstract 5001, the SWOG S1216 trial of TAK-700, compared to bicalutamide, with ADT in mCSPC. She highlighted that this trial stratified patients according to “minimal” and “extensive” disease burden, a characteristic that has not been used in prior trials and makes it somewhat difficult to compare to other trials in this disease space.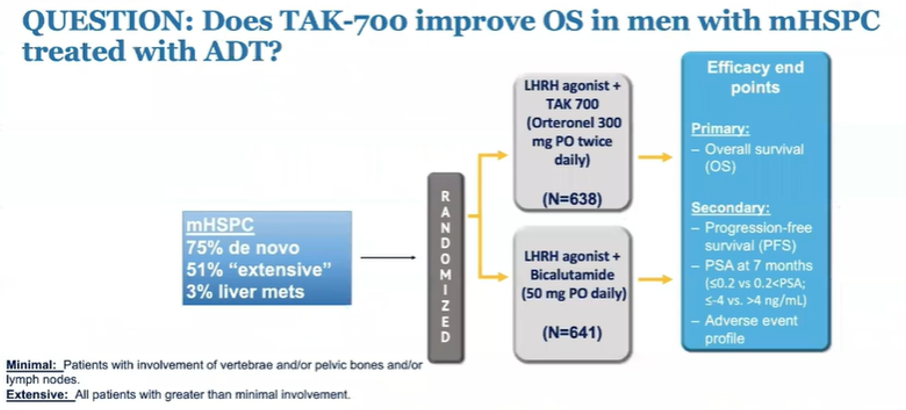
She highlighted that the observed progression-free survival benefit (of approximately 2 years) was similar to that seen in PEACE-1. However, overall survival results are available for SWOG S1216, demonstrating no significant benefit. She did emphasize though that the absolute overall survival seen in both the experimental arm and control arm was higher than many prior studies.
She emphasized that this study, along with previous publications ELM-PC4 and ELM-PC5 in the mCRPC setting, demonstrate that TAK-700 is not equivalent to abiraterone, failing to demonstrate benefit in settings that abiraterone has shown to increase overall survival. She concluded that this study is a well designed randomized controlled trial with a better than usual representation of African American men. However, it is somewhat difficult to compare to other randomized controlled trials in this disease space due to different risk stratification groups. Overall, she concluded that these data do not support using TAK-700.
Finally, Dr. Horvath discussed Dr. Gillessen’s presentation of safety data from PEACE-3, assessing the importance of bone protective agents in this trial of enzalutamide with or without radium-223 in mCRPC.
She highlighted that the intervention in question in this present analysis is the independent data monitoring committee mandate for use of bone-targeting agents.
She emphasized that it was good to see that fracture rates improved once the use of bone protective agents was mandated and increased.
These data further show that rates of fracture are significant in patients who do not receive bone targeting agents (15.6%), decreasing to 2.6% among those receiving bone targeting agents. This substantial decrease in fracture risk outweighs, in her view, the risks of osteonecrosis of the jaw (ranging from 1-5%).
Dr. Horvath emphasized that this analysis of the PEACE-3 trial emphasizes the importance of independent data monitoring committees to improve patient outcomes. However, the data presented so far do not represent a randomized comparison and are limited by small numbers and lack of toxicity data.
Taken together, she emphasized that none of these presented abstracts support a change in current guideline-recommended treatment approaches. Instead, highlighting data from ENZAMET, she emphasized the wide variation in outcomes for patients with mCSPC.
Thus, ongoing work is needed to better risk-stratify patients. There are ongoing efforts from both the ICECaP group and the STOPCAP M1 groups to translate data on prognostic factors in advanced prostate cancer into more personalized treatment recommendations.
However, there is also ongoing work assessing biological, genomic, and metabolomic data from several of the randomized trials of treatment in mCSPC. This, in combination with clinical risk stratification, will help evolve towards more personalized care.
Dr. Horvath then concluded that, on the basis of the data presented today, triplet therapy improves radiographic progression-free survival without proven benefits in overall survival. Thus, she suggested that this does not change the current standard of care where abiraterone is already an accepted option. Further, data from SWOG S1216 demonstrate that TAK-700 has no role in the treatment of patients with mCSPC and from PEACE-3 demonstrate the importance of bone protective agents in mCRPC. However, personalized treatment requires the development of clinical and biologically defined risk groups.
Presented By; Lisa Horvath, MBBS, Ph.D., Director of the Department of Medical Oncology at Chris O’Brien Lifehouse
Written By: Christopher J.D. Wallis, MD, Ph.D., Urologic Oncology Fellow, Vanderbilt University Medical Center, Twitter: @WallisCJD at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting #ASCO21, June, 4-8, 2021


