(UroToday.com) During the 2023 American Society of Clinical Oncology (ASCO) annual meeting Dr. Andrea Necchi discusses 3 oral abstracts with an emphasis on Striking a Balance between Efficacy and Safety for advanced urothelial carcinoma.
He discussed the following 3 abstracts, which are covered separately by Urotoday.
- Abstract 4512: Impact of histology on the efficacy and safety of pembrolizumab (pembro) monotherapy for advanced urothelial carcinoma (UC) in the phase 3 KEYNOTE-045 and KEYNOTE-361 trials
Patrizia Giannatempo, MD | Fondazione IRCCS Istituto Nazionale dei Tumori
- Abstract 4513: Outcomes by complete response to first-line pembrolizumab or platinum-based chemotherapy in advanced urothelial carcinoma (UC) in KEYNOTE-361
Ozgur Ozyilkan, MD | Adana Baskent Üniversitesi
- Abstract 4514: Safety analysis by UGT1A1 status of TROPHY-U-01 cohort 1, a phase 2 study of sacituzumab govitecan (SG) in patients (pts) with metastatic urothelial cancer (mUC) who progressed after platinum (PT)-based chemotherapy and a checkpoint inhibitor (CPI)
He began by focusing on abstract 4513, which reported longer-term outcomes of patients treated with first line pembrolizumab or platinum based chemotherapy in the KEYNOTE-361 trial who had a complete response.
The basis of this analysis was the landmark phase 3 randomized, open-label KEYNOTE-361 (NCT02853305) study,1 in which patients with untreated, locally advanced, unresectable, or metastatic urothelial carcinoma were randomized to pembrolizumab ± chemotherapy versus chemotherapy in the first line setting. Ultimately, the trial did not meet its prespecified endpoints.
As Dr. Necchi reminded us, KEYNOTE-361 is one of three key phase three trials evaluating the role of chemo-immunotherapy in the first-line setting.

The others were the IMvigor130 and DANUBE trials. In general, this combination has not seen clinically significant improvement in OS, though atezo/chemo did meet its PFS endpoint.
The authors note that 15% in the Pembro+chemo arm, 11% in the pembro monotherapy arm and 12% in the chemotherapy arm had a complete response (CR). Although not formally tested, survival outcomes appeared similar with pembrolizumab monotherapy versus chemotherapy (hazard ratio [HR] 0.92; 95% CI, 0.77-1.11). This post hoc exploratory analysis examined efficacy outcomes in patients with complete response (CR) to pembrolizumab or chemotherapy in KEYNOTE-361.
Dr. Necchi did highlight the fact that cisplatin and carboplatin-treated patients had similar CR rates (14% cisplatin and 11% carboplatin), which again brings up the fact that we need to reassess the efficacy of carboplatin for treating metastatic urothelial carcinoma. he also emphasized the fact that we need to reassess the prognostic factors for frontline IO therapy for metastatic UC, as some patients may benefit from this.
Median OS was NR (95% CI, NR-NR) with pembrolizumab and NR (95% CI, 25.1-NR) with chemotherapy (HR, 0.20 [95% CI, 0.06-0.70]). The estimated 24-month OS rates were 94.1% and 69.5% in the pembrolizumab and chemotherapy arms, respectively.
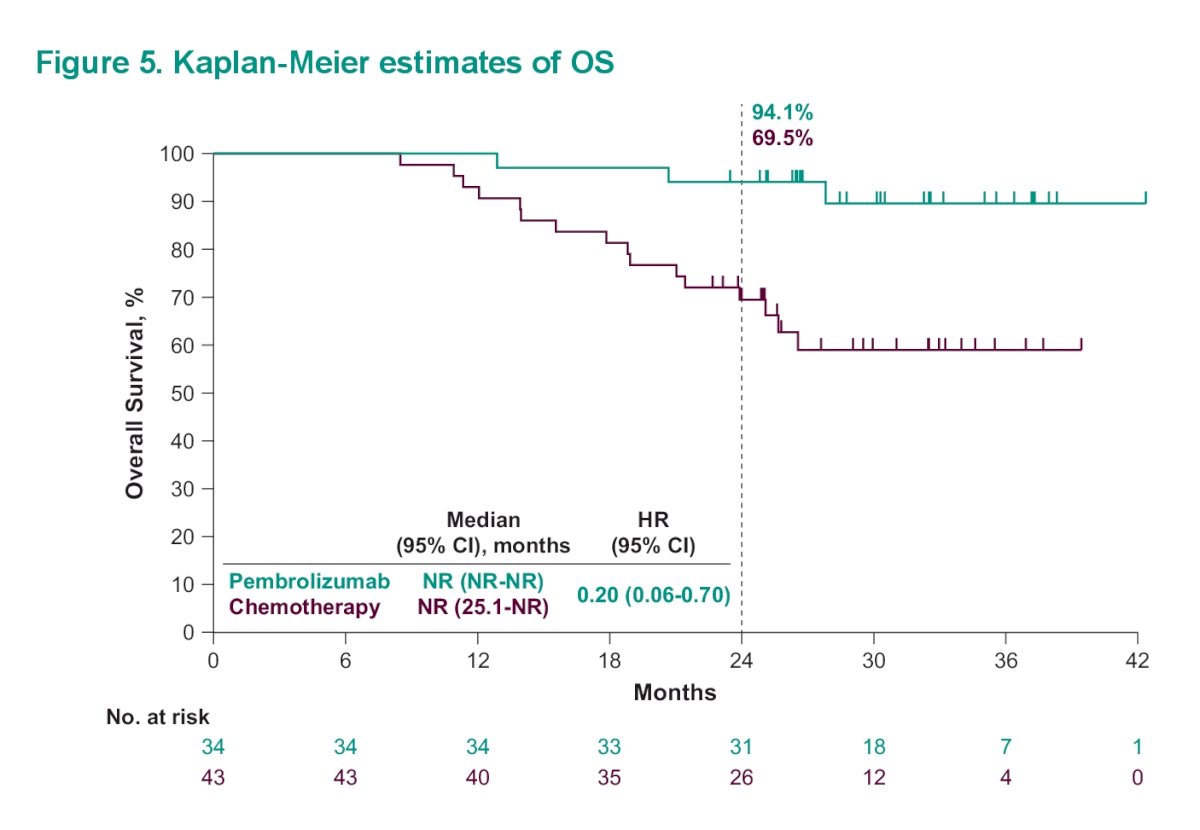
Dr. Necchi then asked is CR an appropriate intermediate endpoint in patients with UC receiving front-line pembrolizumab. He feels it is suitable for de-escalation studies and biomarker discovery. However, it may miss the fact that there are patients with significant durable response that would have benefited from io therapy rather than chemotherapy.
However, do these results help identify the optimal first line therapy for mUC? He isn’t quite so sure about this. there are multiple factors to be considered in decision making including patient/disease features and response to subsequent lines of therapy (ideally replicating first line findings).
Indeed, in posthoc analyses of Keynote-361, he notes the following:
- Combined Keynote-052 and Keynote-361 trials - With pembrolizumab monotherapy, patients with CR/PR at week nine had increased overall survival versus those with stable disease or progressive disease
- pembrolizumab may have had fewer initial responses versus chemotherapy, thought had more durable responses
- in an analysis of subsequent therapies, similar overall survival was observed for the following sequences:
- 1L platinum-based chemo subsequent ICI
- 1L pembro subsequent platinum-based chemo
He notes that ICI response in the second line therapy is the same regardless of the response to chemotherapy in the first line (as seen in Keynote-045 and Javelin Bladder 100). In addition, there remain patients with outstanding durable responses to ICI therapy even in the 2nd-4th line settings.
Unfortunately, the biomarkers to select the patients for upfront ICI therapy are missing – but many are being evaluated.
- Tumor mutational burden (TMB) is well positioned – Keynote-361 data suggests that TMB may be used to select responders. Additional trial data seems to provide similar support to this as a biomarker.

- He notes that there are now real world clinical outcome data that further indicate that TMB high patients have improved OS with ICI therapy
He then moved on to the abstract by Dr. Giannatempo and colleagues, which evaluated the impact of histology on the efficacy and safety of pembrolizumab (pembro) monotherapy for advanced urothelial carcinoma (UC) in the phase 3 KEYNOTE-045 and KEYNOTE-361 trials. We already discussed the KEYNOTE-361 study above, but he reviewed the KEYNOTE-045 briefly. In KEYNOTE-045,2 a study of pembro vs investigator’s choice chemo in the second line setting, pembro again was demonstrated to provide durable antitumor activity.
Efficacy outcomes are summarized below:
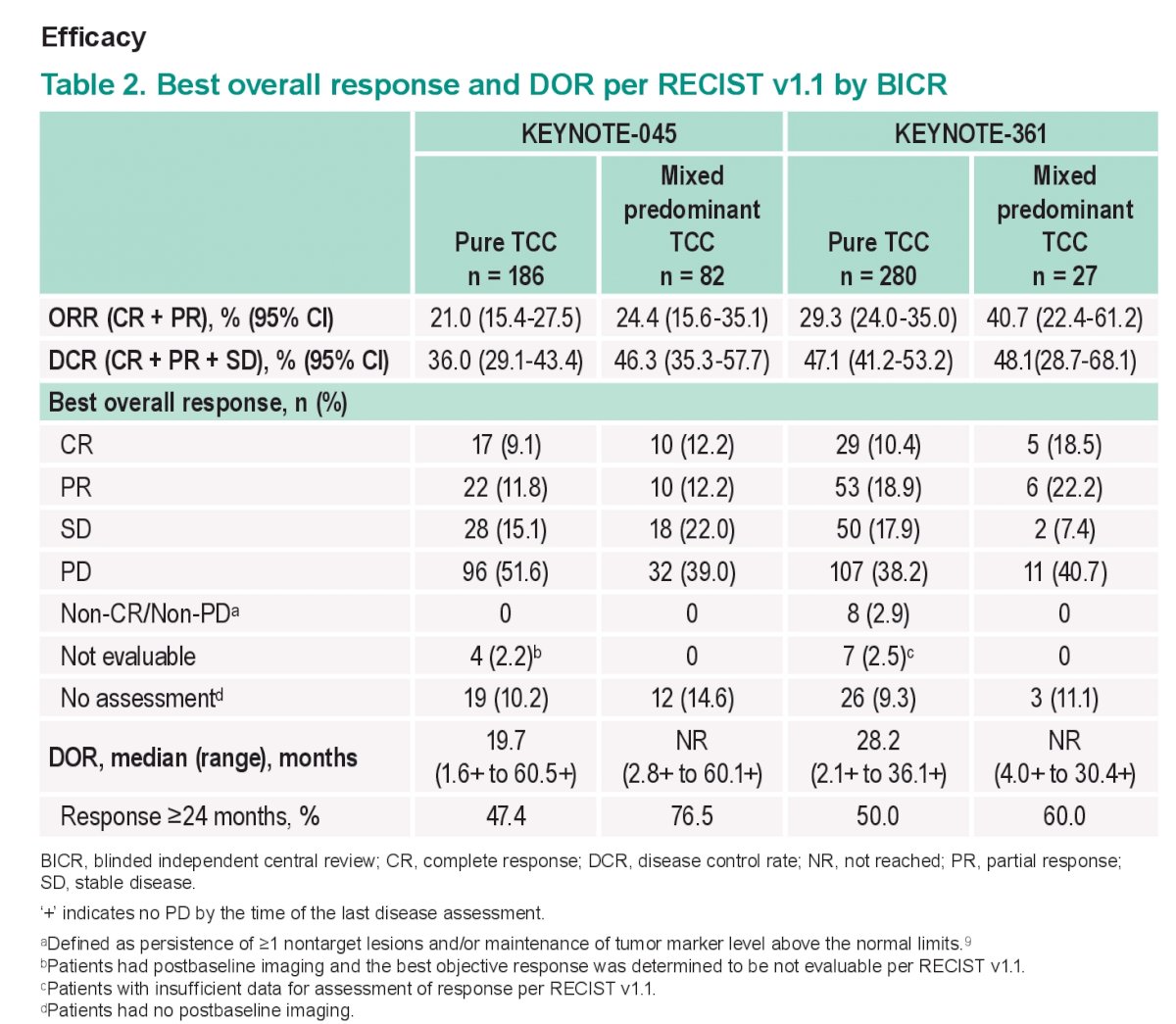
The key take-home points here are that the key efficacy outcomes (PFS, ORR, DOR, and OS) for pembro were generally similar in patients with mixed predominant TC histology and pts with pure TC histology. therefore it would appear that a mixed Histology did not result in significantly different outcomes.
This is reinforced by the overall survival Kaplan Meyer curve seen below:

But he notes that she did not present the distribution of the variant histology subtypes – and unfortunately, parent histology comprises a wide variety of histologic entities. They represent approximately 25% of bladder cancer samples. For clinical trial purposes, a 50% cutoff is typically used to determine if a sample is primarily urothelial histology or primarily variant histology. Therefore, presumably, the patients in these trials had <50% variant histology – which may account for the lack of differences seen.
There is no consensus amongst experts on the role of IO therapy for variant histology urothelial carcinoma – even though data from expanded access programs reveals that response rates are pretty similar to pure UC.
He did note that in his PURE-01 study evaluating neoadjuvant pembrolizumab in patients with MIBC, there was an analysis of patients with predominant or pure variant histology (19 patients). In their assessment, there was lower activity at pembrolizumab in this cohort – Only 16% achieved pT0N0 status and 41% had downstaging to pT0-1 N0. however, there were significant heterogeneity in this group. Small cell neuroendocrine tumors were excluded. SCC variant histology seemed to benefit the most from pembrolizumab monotherapy (86% downstaging).
In a 2020 paper,3 Dr. Necchi and colleagues reported on the immune-oncology biomarkers in variant histology bladder cancer using the Foundation Medicine dataset. PDL-1 and TMB were higher in SCC but lower in adenocarcinoma, compared to pure urothelial carcinoma.
The two main questions stemming from this abstract are:
- Are these data sufficiently robust to recommend pembrolizumab use for patients with variant Histology? NOT YET!
- do these data need further investigation in patients with pure/ predominant variant Histology? Yes, and he feels probably in variant squamous cell carcinoma.
The final abstract he discusses was the work by Loriot and colleagues reporting the safety analysis by UGT1A1 status of TROPHY-U-01 cohort 1,4 a phase 2 study of sacituzumab govitecan (SG) in patients (pts) with metastatic urothelial cancer (mUC) who progressed after platinum (PT)-based chemotherapy and a checkpoint inhibitor (CPI)
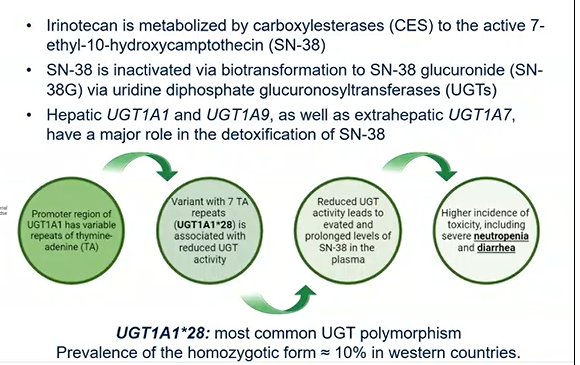
Dr. Necchi highlights the reason to stratify response based on UGT1A1. This is due the fact that hepatic UGT1A1 and UGT1A9 play a major role in the detoxification of SN-38 (which is the major payload of irinotecan). UGT1A1*28 is the most common polymorphism and approximately 10% of patients have the homozygotic form in Western countries.
The study design for TROPHY-U-01 is shown below:
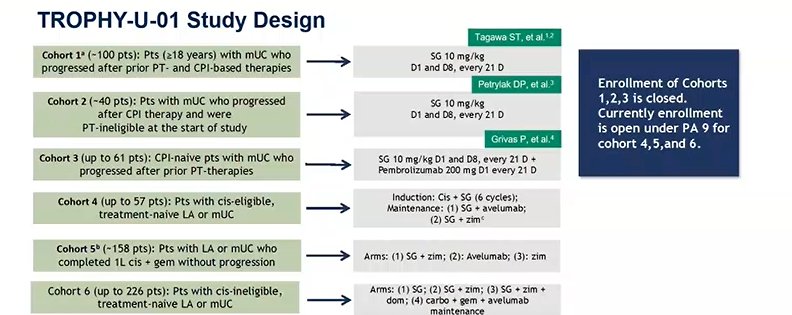
Patients in Cohort 1 were specifically patients that had progressed following platinum-based chemo and a CPI. They received SG 10 mg/kg on D1 and D8 of 21-D cycles. The primary endpoint was ORR per central review by RECIST 1.1. For the purposes of this abstract, post hoc safety analyses were exploratory with descriptive statistics reported.
Dr. Necchi first notes that Cohorts 1-3 all reported similar rates of Grade 3+ TRAEs. There were no major differences depending on stages of use.
In total, 94% of treated patients (n=106) had an evaluable UGT1A1 status, of which 45 were wild type, 47 were heterozygous, and 14 were homozygous.
- Gr ≥3 TRAEs occurred in 62% of wild-type, 60% of heterozygous, and 79% of homozygous patients
Breaking down the types of AEs (any grade):
- Any Gr diarrhea was 53%, 72%, and 71%, respectively
- Any Gr neutropenia was 38%, 55%, and 50%, respectively
- Any Gr anemia was 38%, 32%, and 29%, respectively
Focusing on Grade 3+ AEs:
- Diarrhea: 4%, 15%, and 7%, respectively;
- Neutropenia: 31%, 36%, and 50%, respectively
- Anemia: 13%, 19%, and 29%
TRAEs leading to SG discontinuation occurred in 7%, 6%, and 14% of pts with wild-type, heterozygous, and homozygous status, respectively.
The fundamental questions that he chose to answer or address or as follows:
- what is the most appropriate use of G-CSF (primary vs secondary prophylaxis)?
- Primary Prophylaxis is Indicated in correct protocols for patients with high risk of developing FN per ASCO guidelines
- what is the timing of onset of severe TRAEs – in his experience, usually after C1D8.
- Can patient adherence be optimized with alternative schedules, maybe every 2 weeks?
- How can the risk of her deadly combination of severe neutropenia and diarrhea be minimized?
- careful consideration of patients with nephrostomy tubes, history of IBD, etc.
Based on all of the above, he feels the UGT1A1 test maybe advised in addition to other selection criteria and G-CSF use recommendations to optimize SG delivery in potentially curable settings.
But, genotype-guided dosing of SG is currently not supported by the data.
Presented by: Andrea Necchi, MD Fondazione IRCCS Istituto Nazionale dei Tumori
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Associate Professor of Urology, University of California, Davis, @tchandra_uromd @UCDavisUrology on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
References:
- Powles T., Csőszi T., Özgüroğlu M. et al. "Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial." Lancet Oncology. 2021. 931-945.
- Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Nam K, Frenkl TL, Perini RF, de Wit R, Bajorin DF. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019 Jun 1;30(6):970-976. doi: 10.1093/annonc/mdz127. PMID: 31050707; PMCID: PMC6594457.
- Necchi A, Madison R, Raggi D, Jacob JM, Bratslavsky G, Shapiro O, Elvin JA, Vergilio JA, Killian JK, Ngo N, Ramkissoon S, Severson E, Hemmerich AC, Huang R, Ali SM, Chung JH, Reddy P, Miller VA, Schrock AB, Gay LM, Alexander BM, Grivas P, Ross JS. Comprehensive Assessment of Immuno-oncology Biomarkers in Adenocarcinoma, Urothelial Carcinoma, and Squamous-cell Carcinoma of the Bladder. Eur Urol. 2020 Apr;77(4):548-556. doi: 10.1016/j.eururo.2020.01.003. Epub 2020 Jan 17. PMID: 31959546.
- Tagawa ST, Balar AV, Petrylak DP, Kalebasty AR, Loriot Y, Fléchon A, Jain RK, Agarwal N, Bupathi M, Barthelemy P, Beuzeboc P, Palmbos P, Kyriakopoulos CE, Pouessel D, Sternberg CN, Hong Q, Goswami T, Itri LM, Grivas P. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J Clin Oncol. 2021 Aug 1;39(22):2474-2485. doi: 10.1200/JCO.20.03489. Epub 2021 Apr 30. PMID: 33929895; PMCID: PMC8315301.


