(UroToday.com) The 2023 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL between June 2nd and June 6th was host to a prostate, testicular, and penile cancers poster discussion session. In the discussant presentation, Dr. Ravi Madan discussed quality in the context of quantity: evaluating treatment intensification. He discussed three abstracts presented in this session:
- Patient-reported quality of life and survival outcomes: Analysis of ECOG-ACRIN E3805 chemohormonal androgen ablation randomized trial (CHAARTED) in prostate cancer
- Patient-reported outcomes among men receiving talazoparib + enzalutamide versus placebo + enzalutamide as first-line treatment for metastatic castration-resistant prostate cancer: Results from a phase 3 study (TALAPRO-2)
- Health-related quality of life (HRQoL) and pain outcomes for patients with metastatic castration-resistant prostate cancer who received abiraterone and olaparib versus abiraterone and placebo in the phase III PROpel trial
Dr. Madan first discussed the following abstract: “Patient-reported quality of life and survival outcomes: Analysis of ECOG-ACRIN E3805 chemohormonal androgen ablation randomized trial (CHAARTED) in prostate cancer”.
In this study, patients were randomized in a 1:1 fashion to ADT plus docetaxel (6 cycles of 75 mg/m2 every 3 weeks) or ADT alone. QoL was evaluated using the Functional Assessment of Cancer Therapy – Prostate (FACT-P) at 3-month intervals. The association between QoL and OS was assessed using Kaplan Meier curves, with comparisons performed using the log-rank test, and Cox proportional hazards modeling, adjusted for disease/patient characteristics. This analysis included a total of 790 patients, with 397 and 393 in the intervention and control arms, respectively.
This study demonstrated that a higher 3-month FACT-P score was associated with improved OS outcomes on multivariable modeling (HR: 0.76, 95% CI: 0.58 – 1.00, p=0.05), irrespective of treatment arm or disease volume, as demonstrated below:
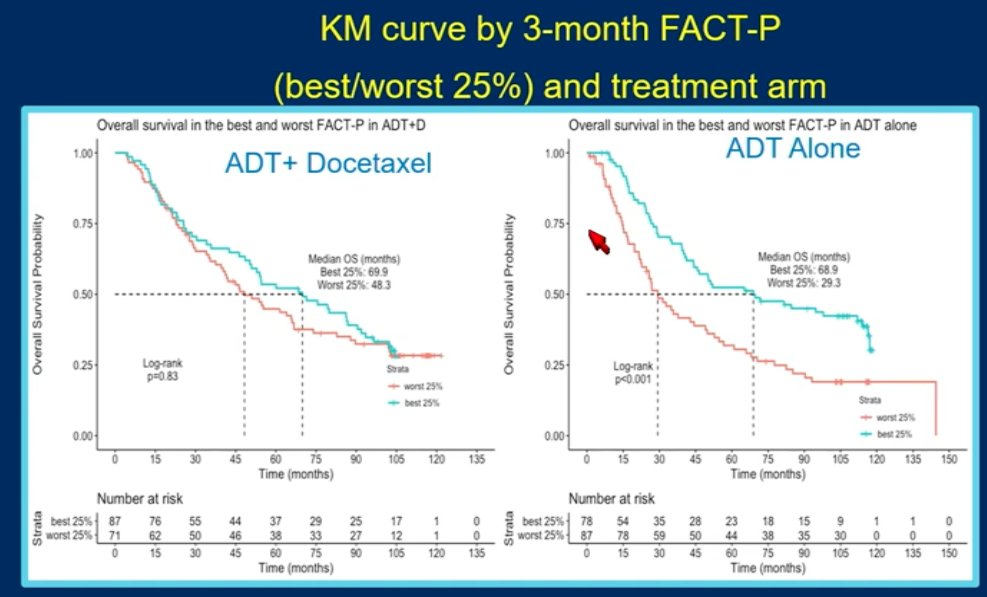
Dr. Madan next discussed the following abstract: “Patient-reported outcomes among men receiving talazoparib + enzalutamide versus placebo + enzalutamide as first-line treatment for metastatic castration-resistant prostate cancer: Results from a phase 3 study (TALAPRO-2)”. The study design was as follows:
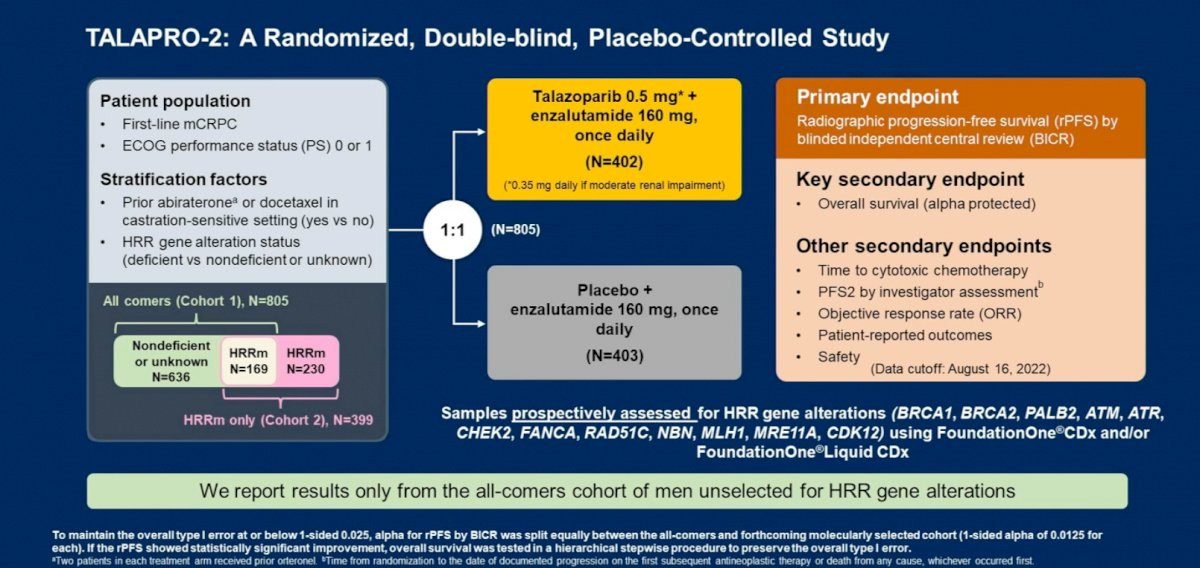
In this report, Dr. Agarwal and colleagues reported PROs from TALAPRO-2. PROs were assessed at baseline, at scheduled visits (every 4 weeks up to week 53, thereafter every 8 weeks) until progression, and at safety (at treatment discontinuation) and long-term follow-up visits. PRO instruments included the EORTC QLQ-C30 and its prostate cancer module, QLQ-PR25.
Using the EORTC QLQ-C30, the time-to-definitive deterioration in global health status (GHS)/QoL was significantly longer with combination talazoparib plus enzalutamide, compared to placebo plus talazoparib (HR: 0.78, 95% CI: 0.62 – 0.99, p=0.038).
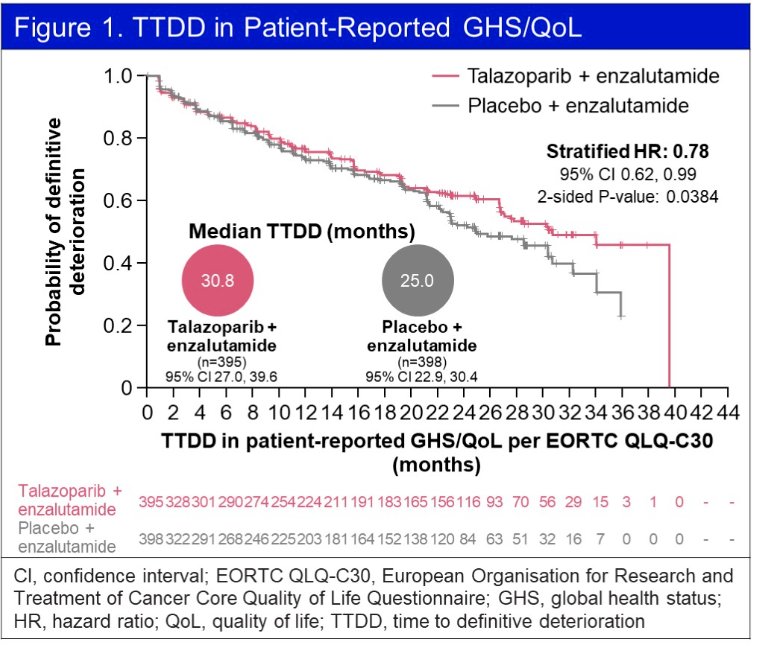
The treatment effect on estimated mean change in GHS/QoL score from baseline similarly favored the intervention arm, although the difference was not clinically meaningful. Furthermore, no significant difference in functional scales were observed between the two arms. There were no clinically meaningful differences in any of the individual symptom scales.
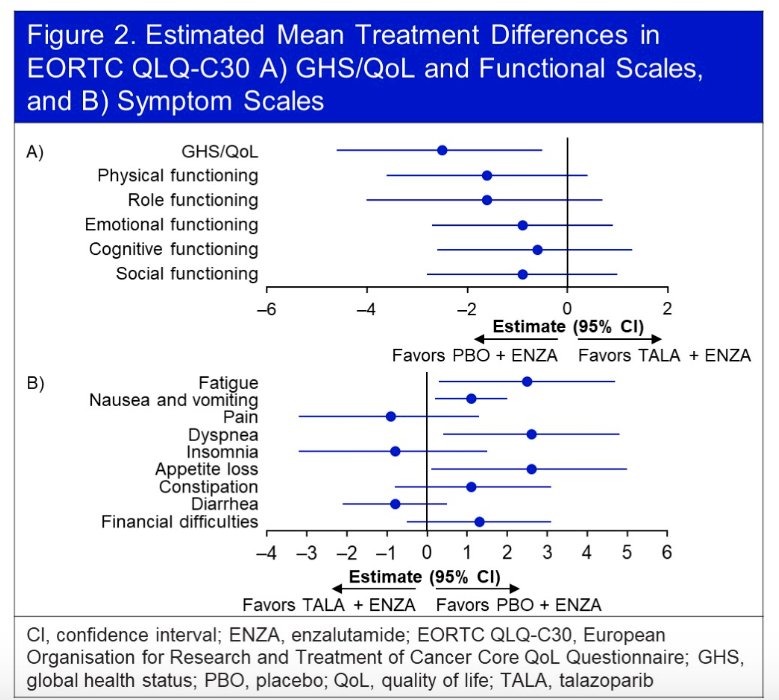
Using the EORTC QLQ-PR25 questionnaire, time-to-definitive deterioration of urinary symptoms was non-significantly prolonged in the intervention arm (HR: 0.76, 95% CI: 0.54 - 1.06, p=0.11).
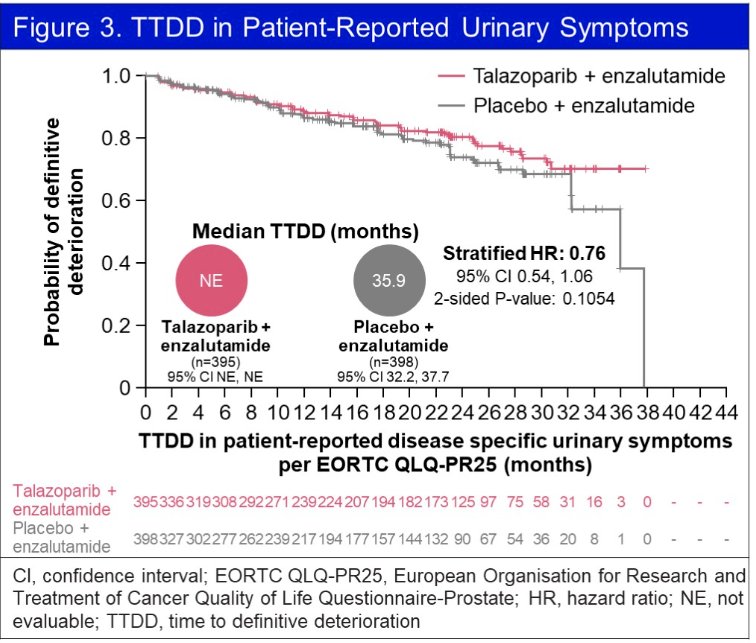
No significant differences were observed in any functional or symptom scales.
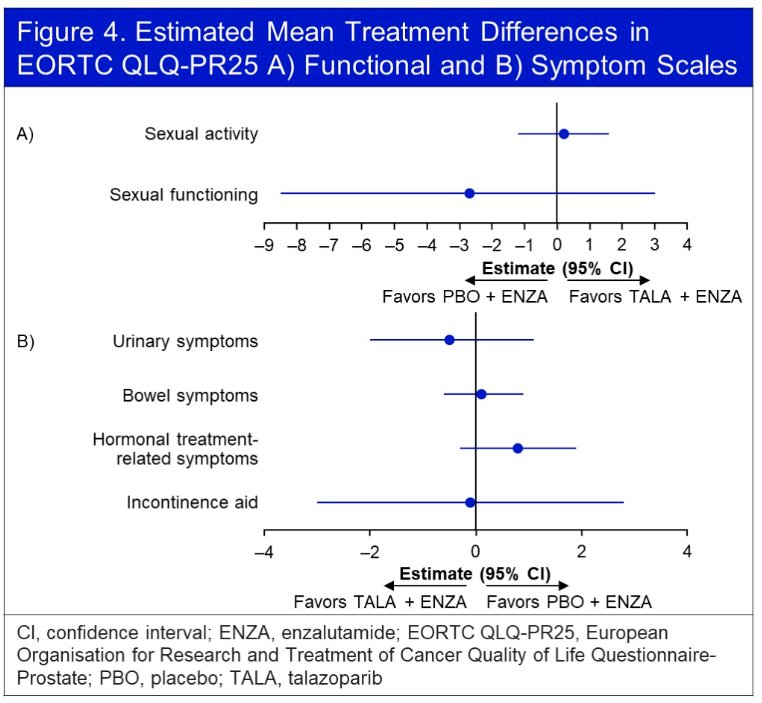
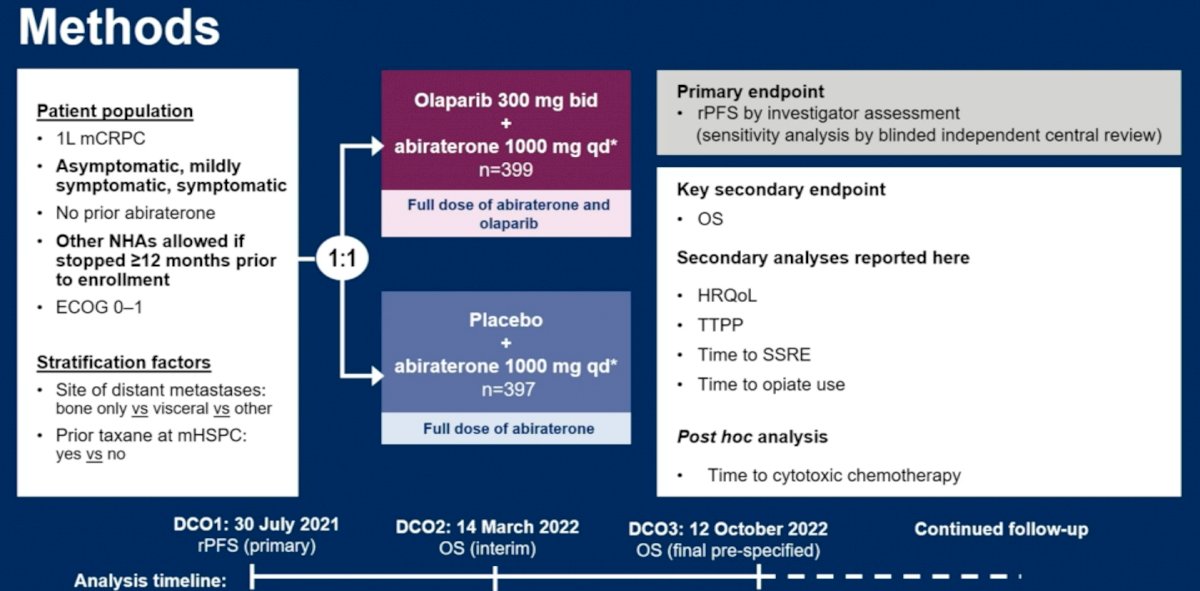
The third abstract discussed by Dr. Madan was: “Health-related quality of life (HRQoL) and pain outcomes for patients with metastatic castration-resistant prostate cancer who received abiraterone and olaparib versus abiraterone and placebo in the phase III PROpel trial”.
The study design was as follows:
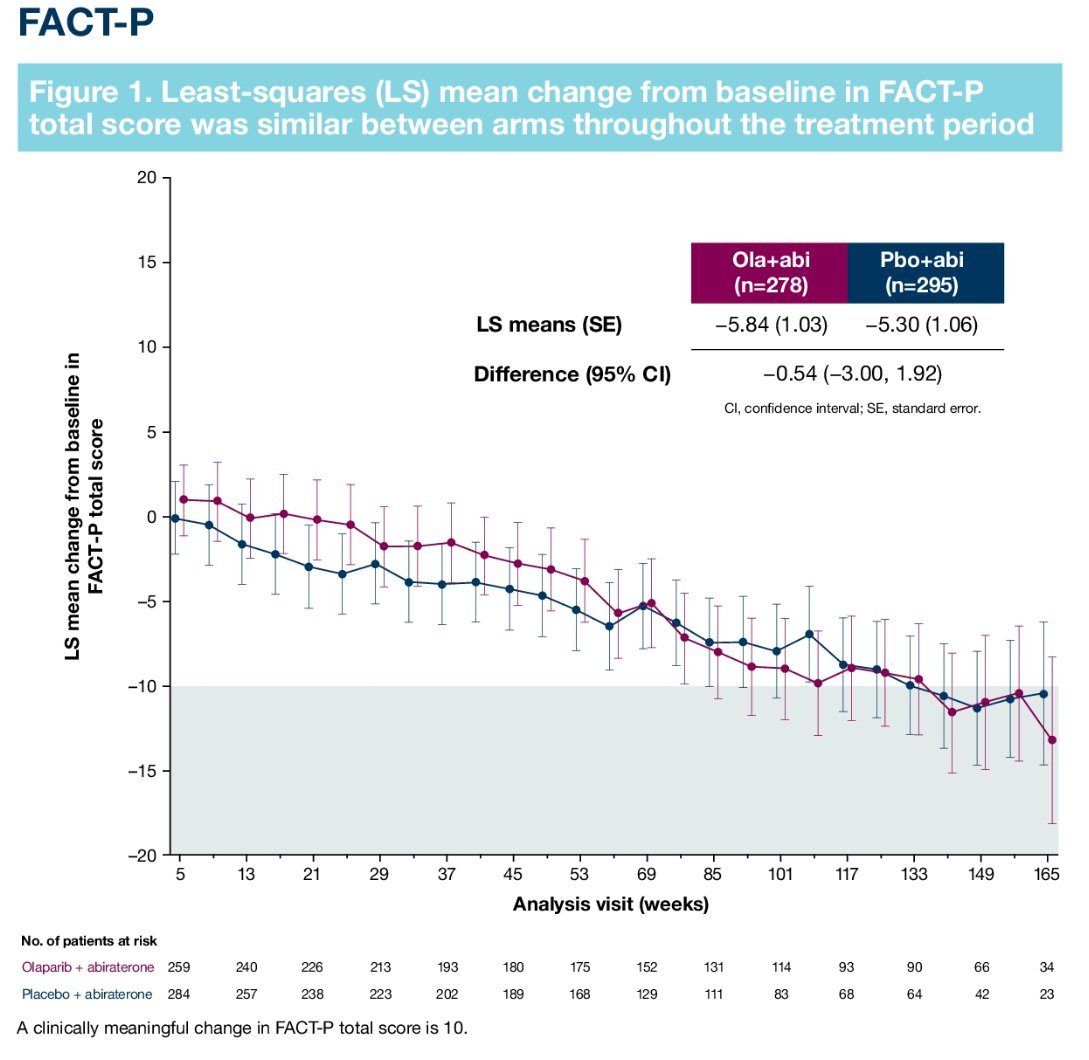
In this trial, no significant differences were observed in FACT-P assessed HRQoL scores between the two arms:
Similarly, pain scores and time-to-pain progression rates were non-significantly different between the two arms:
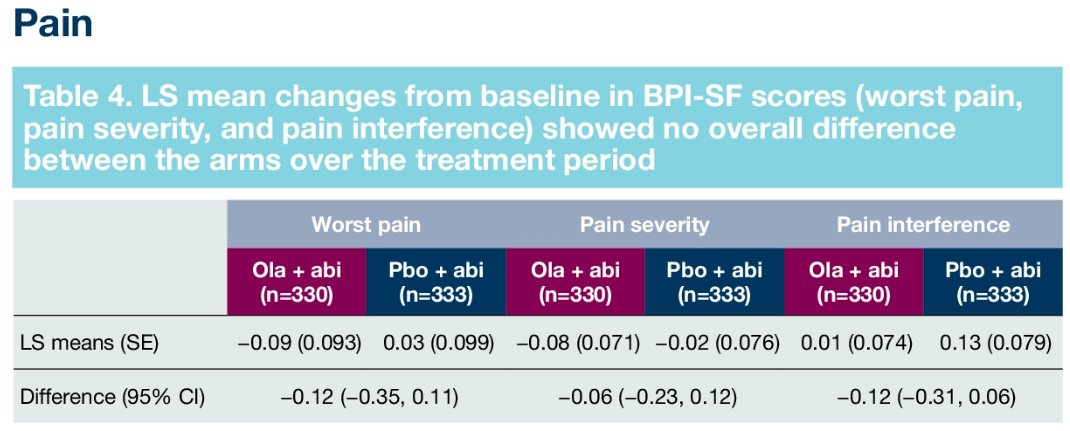
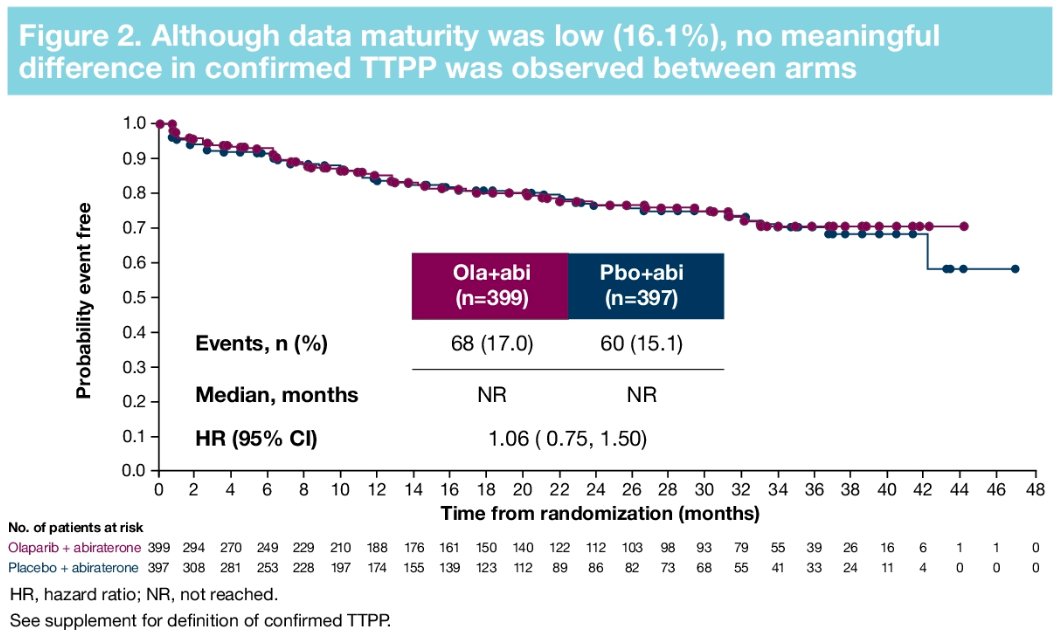
Furthermore, there were no significant differences in time to first symptomatic skeletal-related event (HR: 0.82, 95% CI: 0.55 – 1.22) or time to opiate use (HR: 1.21, 95% CI: 0.82 – 1.79). in the overall cohort.
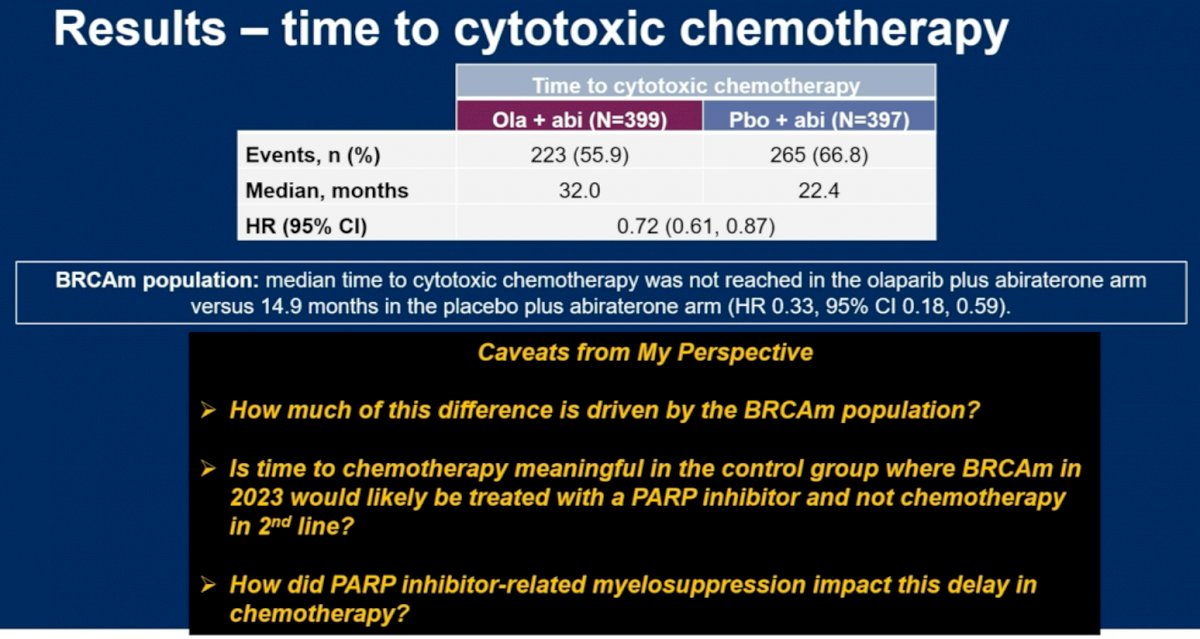
Dr. Madan did note however that time to cytotoxic chemotherapy was significantly prolonged in the combination arm of olaparib + abiraterone: 32 versus 22.4 months (HR: 0.72, 95% CI: 0.61 – 0.87). He posed the following questions however:
Dr. Madan concluded by noting that PROs and QoL assessments best inform clinical practice when:
- Two or more treatment options have negligible toxicity
- Two or more treatment options appear to have equivalent toxicity
- This appears to be relevant when considering the toxicity profile of combination androgen receptor signaling inhibitors plus PARP inhibitors from TALAPRO-2 and PROpel and the need to balance reported toxicity profiles with the PROs from these trials
Presented by: Ravi Amrit Madan, MD, Senior Clinician, National Cancer Institute (NCI), Bethesda, MD
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 2 – Tues, June 6, 2023.
ASCO 2023: Patient Reported Quality of Life and Survival Outcomes: Analysis of ECOG-ACRIN E3805 Chemohormonal Androgen Ablation Randomized Trial (CHAARTED) in Prostate Cancer
ASCO 2023: Patient-Reported Outcomes Among Men Receiving Talazoparib + Enzalutamide vs Placebo + ENZA as First-Line Treatment for mCRPC: Results from a Phase 3 Study (TALAPRO-2)
ASCO 2023: Health-Related Quality of Life and Pain Outcomes for Patients with mCRPC Who Received Abiraterone and Olaparib Vs Abi and Placebo in the Phase III PROpel Trial


