(UroToday.com) The 2024 American Society of Clinical Oncology (ASCO) annual meeting held in Chicago, IL was host to the session Advancing Prostate Cancer Care: Treatment Approaches to Precision Medicine, Biomarker Innovations, and Equitable Access. Dr. David James VanderWeele discusses a practical approach to genetic testing and treatment selection in prostate cancer (PCa).
Dr. VanderWeele began his presentation by recounting the case of a 72-year-old male patient diagnosed with high-volume de novo metastatic hormone-sensitive prostate cancer (mHSPC), who had a family history of prostate cancer in his father and brother. Somatic genetic testing of this patient revealed mutations in BRCA2 (N1747fs1) with a variant allele frequency of 34% and TP53 (R249K), while germline testing showed mutations in BRCA2 (p.Asn1747) and surprisingly an alteration in HOXB13 (p.Gly84Glu). He centered his discussion on how genetic testing enhanced the patient's treatment selection.
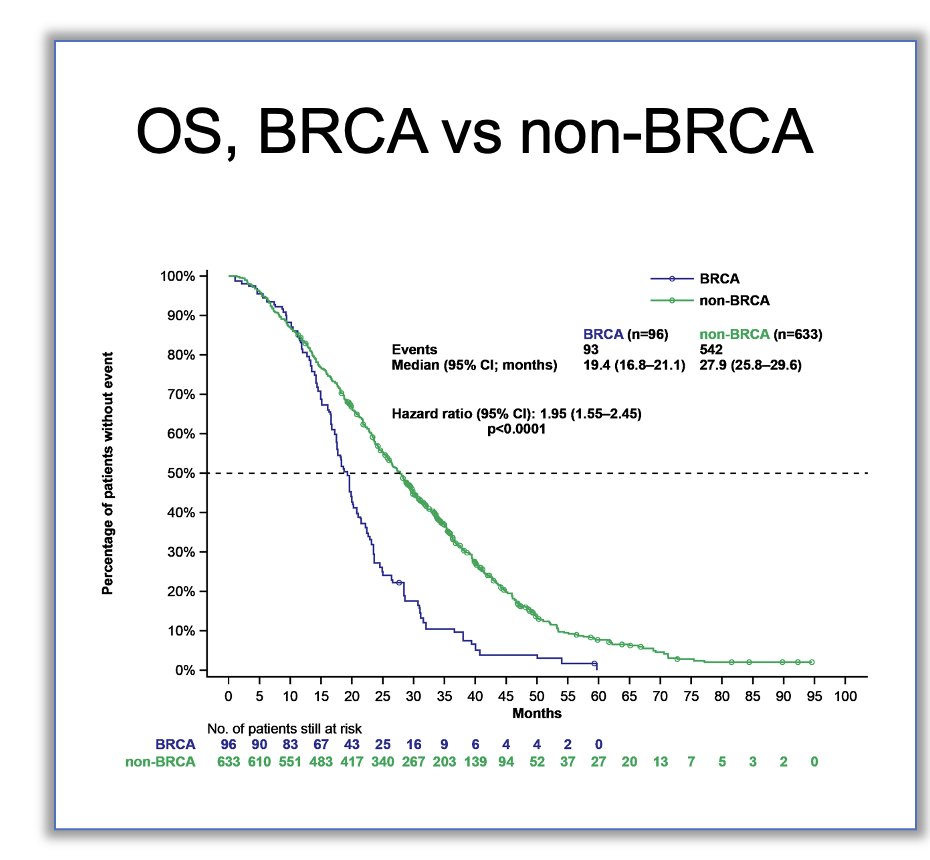
BRCA2 mutations have been linked to an increased risk of prostate cancer diagnosis, up to two times higher, with some studies indicating a 60% lifetime risk of PCa when this gene mutation is present and that’s why cascade testing is recommended. Additionally, BRCA2 mutations are associated with poorer prognosis and overall survival (OS) compared to non-BRCA patients (Refer to graphic below). Dr. VanderWeele revisited the patient discussed earlier, noting that despite receiving treatment with a Triplet regimen (Darolutamide + Docetaxel + ADT), the patient progressed at 1 year of follow-up. He pointed out data from the ARASENS trial,1 where 60% of patients remained progression-free at 4 years of follow-up, likely attributed this patient's progression to his BRCA2 mutation. Moreover, in the CAPTURE study, significantly, OS was worse in BRCA1/2 mutated patients compared to patients with non-BRCA mutations (HR: 1.95, 95% CI: 1.55 – 2.45, p<0.001).2
Furthermore, Dr. VanderWeele highlighted that BRCA2 mutations also predict the response to PARP inhibitors, as evidenced by studies such as TALAPRO23 as well as the response to platinum-based agents (case series) and is currently being tested in the Veterans Affairs (VA) system in the COBRA trial.
Notably, the patient presented by Dr VanderWeele, also had a HOXB13 germline mutation, a homeobox transcription factor gene crucial in prostate development, which has been linked to an elevated risk of hereditary prostate cancer. It's been found to confer up to a 65% lifetime risk of prostate cancer. However, to date, there isn't a clear association between HOXB13 mutations and prognosis or treatment response.4
A practical strategy for genetic testing involves initiating somatic testing early in the diagnostic process. One compelling rationale for this approach is the documented 30% failure rate associated with tissue testing, a rate that tends to increase with the age of the tissue sample; this is very important considering the natural history of PCa, and patients tend to relapse with metastatic disease many years after their diagnosis. Furthermore, genetic test results offer valuable insights into predicting response to AR-targeted therapy and can inform prognostic assessments. Patients harboring alterations in homologous recombination repair (HRR) and mismatch repair (MMR) pathways are often associated with higher-risk disease and may experience more rapid disease progression compared to those without such genetic alterations. Therefore, early somatic testing can provide critical information to guide treatment decisions and prognostic evaluations.
When considering the use of cell-free DNA (cfDNA), it's important to note that cfDNA closely mirrors tissue sequencing results only when the tumor fraction is high. A study comparing circulating tumor DNA (ctDNA) alterations with exome sequencing data from matched tissue samples quantified the concordance of mutations and copy number alterations in metastatic castration-resistant prostate cancer (mCRPC) patients.5 The findings revealed a high correlation in copy number profiles between matched liquid and solid biopsies. More importantly, when the tumor fraction is high cfDNA is similar to tissue sequencing (refer to figure below).

Dr. VanderWeele emphasized that based on these findings, it is advisable to reserve cfDNA analysis for patients with a high burden of active disease, such as those with mCRPC. This targeted approach ensures that cfDNA testing is utilized effectively in patients where its accuracy aligns with clinical needs.
Factors that have been associated with successful cfDNA sequencing are:
- High PSA and alkaline phosphatase
- Short PSA doubling time
- High tumor burden
- Castration resistant disease
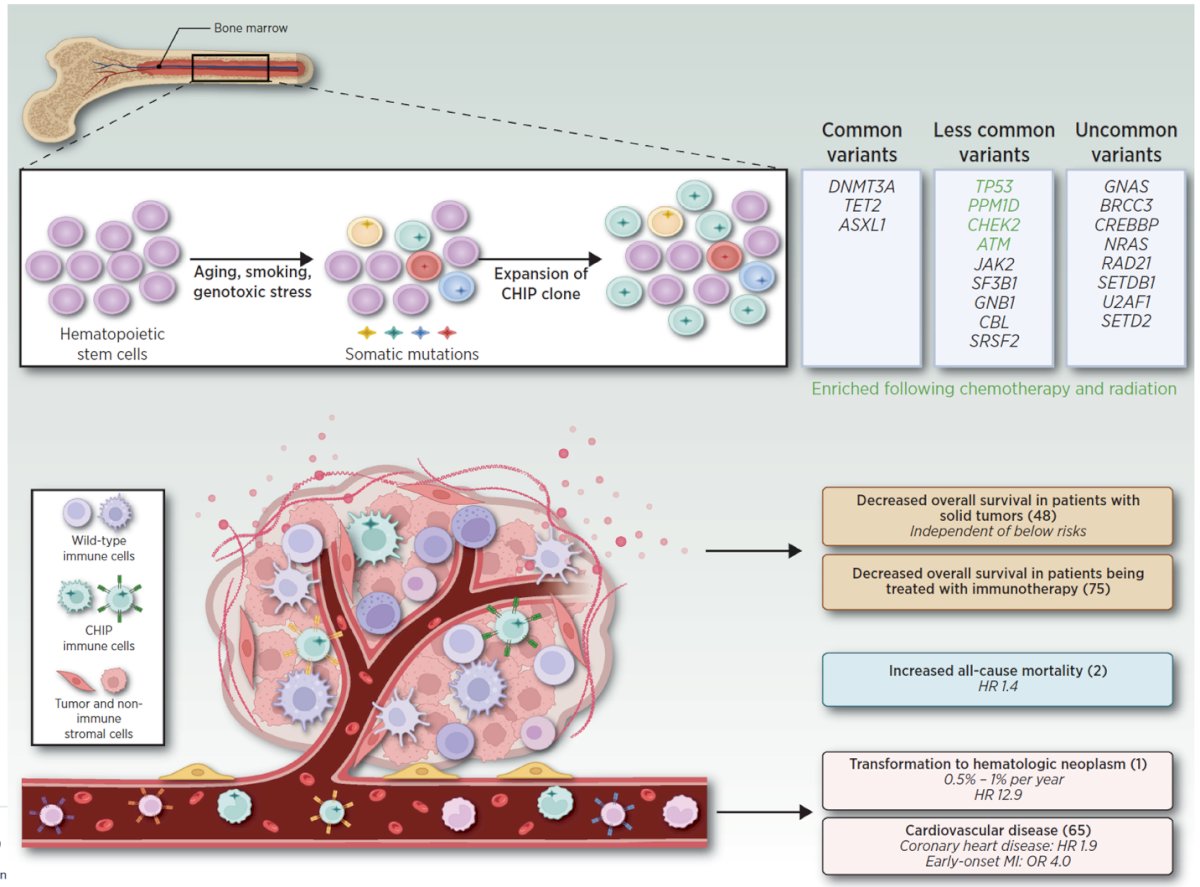
Furthermore, we should avoid mistaking clonal hematopoiesis for tumor DNA. Clonal hematopoiesis of indeterminate potential (CHIP) is characterized by the expansion of hematopoietic cells harboring leukemia-associated somatic mutations in otherwise healthy people and occurs in at least 10% of adults over 70 years. CHIP can be mistaken for mutations in prostate cancer cells, and this includes potentially actionable genes like CHEK2, ATM, TP53.6 The presence of CHIP can also be associated with the development of hematological malignancies, cardiovascular disease, and decreased survival.
Dr. VanderWeele further discussed that alterations in the SPOP gene are linked to favorable outcomes with androgen receptor (AR) therapies. SPOP, which stands for "speckle type BTB/POZ protein," serves as an adaptor protein within the CUL3-RBX1 E3 ubiquitin ligase complex. This complex is involved in binding multiple oncogenic proteins for ubiquitination and subsequent proteasomal degradation. Loss-of-function mutations in SPOP are considered an early event in prostate tumorigenesis and are associated with increased AR signaling.
A study utilizing population-level data of patients with metastatic hormone-sensitive prostate cancer (mHSPC) revealed that SPOP mutations (n=6) were correlated with improved outcomes to androgen deprivation therapy (ADT) plus AR-pathway inhibitor (ARPI) depicted below. This finding underscores the potential clinical significance of SPOP mutations as a predictive biomarker for treatment response in prostate cancer.7

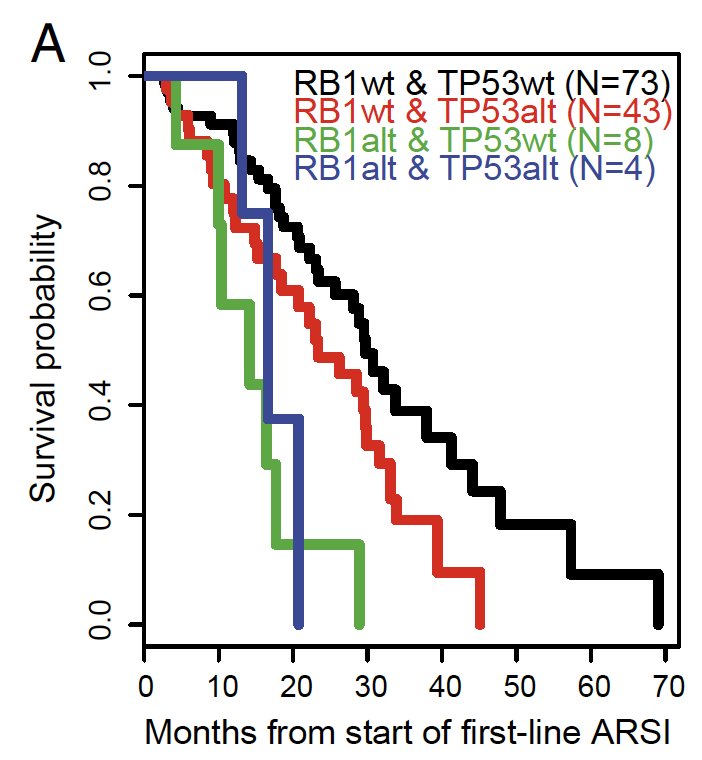
Alterations in PTEN, RB1, TP53 are associated with worse outcomes on AR therapies. In a study of 429 patients with mCRPC, RB1 alteration was significantly associated with poor survival, and TP53 was associated with shorter time on treatment with an ARPI, figure below.8
In patients with mCRPC and alterations in PTEN, RB1, TP53 there is a benefit from carboplatin added to cabazitaxel in progression free survival (HR 0.35, p=0.00014), as demonstrated by a large phase 2 study from the MD Anderson Cancer Centre.9
Bottom of Form

Dr. VanderWeele emphasized the significance of prioritizing PARP inhibitors over other therapy options in patients with BRCA1/2 mutations. Approximately 2% of PCa patients would have a BRCA1 mutation and 10% a BRCA2 mutation, which are approximately half somatic and half germline. These mutations have been associated with improved radiological progression-free survival (rPFS) in three trials involving patients with metastatic castration-resistant prostate cancer (mCRPC) treated with PARPi (See summary table below). In particular, he highlighted the remarkable nearly 80% improvement in rPFS (HR 0.20) observed in the TALAPRO2 trial compared to placebo plus Enzalutamide.

Despite having FDA approval for Olaparib monotherapy and Enzalutamide+talazoparib for other HRR alterations, Dr. VanderWeele advised prioritizing other therapies before PARP inhibitors in patients with non-BRCA1/2 homologous recombination repair (HRR) mutations. This recommendation stems from the observation that the benefit in terms of radiological progression-free survival (rPFS) with PARP inhibitors is not as pronounced in these patients compared to those with BRCA1/2 mutations. However, he noted exceptions for certain mutations such as CKD12 and PALB2, where the efficacy of PARP inhibitors may warrant consideration earlier in the treatment plan.

Lastly, he discussed MMR alterations which are less frequent (~ 3%) including microsatellite instability high (MSI-H), high tumor burden, and mismatch repair deficiency are associated with a significant PSA response rate (50-75%) when patients with mCRPC harboring these 3 alterations are treated with immune checkpoint inhibitors (Pembrolizumab).10
Furthermore, he discussed that when looking at a genetic testing report this is the way he usually approaches different alterations:
- SPOP - AR therapies
- PTEN, RB1, TP53 - cytotoxic therapies, including carboplatin
- BRCA2, BRCA1, PALB2, CDK12 - PARP inhibitors
- MSH2, MSH6, PMS2 (and MSI-H, High TMB) – pembrolizumab
- Other HRR genes - PARP inhibitors after using other standard therapies
Currently, the indications for germline genetic testing in PCa are:
- Stage IV disease
- Localized that is high risk, or intermediate risk with intraductal/cribiform patterns
- Family history
- Somatic testing showing alteration in a common germline gene with allele frequency 20-80%
Dr. VanderWeele underscored the significance of integrating germline testing with somatic testing, particularly for patients meeting specific criteria. He cited a study involving 2,023 cancer patients who underwent tumor DNA sequencing followed by germline testing. The findings revealed that 30% of patients carried pathogenic germline variants, with 8% of these variants undetected through somatic testing, equating to approximately 2-3% of all patients.11 Consequently, despite negative somatic test results, germline testing should be contemplated for patients exhibiting the outlined indications
Dr VanderWeele, wrapped up his presentation by saying:
- We should offer genetic testing early especially in hormone sensitive prostate cancer
- Cell-free DNA should be reserved for patients with a high burden of active disease, where it closely mirrors tissue sequencing
- Genomic tests can predict response to AR-targeted therapy
- PARP inhibitors should be prioritized highly for BRCA2, BRCA1, CDK12, and PALB2 (but not other HRR genes)
Presented by: David James VanderWeele, MD, PhD, Associate Professor in Medicine - Hematology/Oncology at the Robert H. Lurie Comprehensive Cancer Center, Northwestern University Feinberg School of Medicine
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, May 31 – Tues, June 4, 2024.
References:
- Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, Kopyltsov E, Park CH, Alekseev B, Montesa-Pino Á, Ye D, Parnis F, Cruz F, Tammela TLJ, Suzuki H, Utriainen T, Fu C, Uemura M, Méndez-Vidal MJ, Maughan BL, Joensuu H, Thiele S, Li R, Kuss I, Tombal B; ARASENS Trial Investigators. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142. doi: 10.1056/NEJMoa2119115. Epub 2022 Feb 17. PMID: 35179323; PMCID: PMC9844551.
- Olmos D, Lorente D, Alameda D, et al. Treatment patterns and outcomes in metastatic castration-resistant prostate cancer patients with and without somatic or germline alterations in homologous recombination repair genes. Ann Oncol. Published online February 27, 2024. doi:10.1016/j.annonc.2024.01.011
- Agarwal N, Azad AA, Carles J, Fay AP, Matsubara N, Heinrich D, Szczylik C, De Giorgi U, Young Joung J, Fong PCC, Voog E, Jones RJ, Shore ND, Dunshee C, Zschäbitz S, Oldenburg J, Lin X, Healy CG, Di Santo N, Zohren F, Fizazi K. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023 Jul 22;402(10398):291-303. doi: 10.1016/S0140-6736(23)01055-3. Epub 2023 Jun 4. Erratum in: Lancet. 2023 Jul 22;402(10398):290. PMID: 37285865.
- Ewing CM, Ray AM, Lange EM, Zuhlke KA, Robbins CM, Tembe WD, Wiley KE, Isaacs SD, Johng D, Wang Y, Bizon C, Yan G, Gielzak M, Partin AW, Shanmugam V, Izatt T, Sinari S, Craig DW, Zheng SL, Walsh PC, Montie JE, Xu J, Carpten JD, Isaacs WB, Cooney KA. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med. 2012 Jan 12;366(2):141-9. doi: 10.1056/NEJMoa1110000. PMID: 22236224; PMCID: PMC3779870.
- Wyatt AW, Annala M, Aggarwal R, Beja K, Feng F, Youngren J, Foye A, Lloyd P, Nykter M, Beer TM, Alumkal JJ, Thomas GV, Reiter RE, Rettig MB, Evans CP, Gao AC, Chi KN, Small EJ, Gleave ME. Concordance of Circulating Tumor DNA and Matched Metastatic Tissue Biopsy in Prostate Cancer. J Natl Cancer Inst. 2017 Dec 1;109(12):djx118. doi: 10.1093/jnci/djx118. PMID: 29206995; PMCID: PMC6440274.
- Reed SC, Croessmann S, Park BH. CHIP Happens: Clonal Hematopoiesis of Indeterminate Potential and Its Relationship to Solid Tumors. Clin Cancer Res. 2023 Apr 14;29(8):1403-1411. doi: 10.1158/1078-0432.CCR-22-2598. PMID: 36454121; PMCID: PMC10106364.
- Swami U, Graf RP, Nussenzveig RH, Fisher V, Tukachinsky H, Schrock AB, Li G, Ross JS, Sayegh N, Tripathi N, Mathew Thomas V, Oxnard GR, Antonarakis ES, Agarwal N. SPOP Mutations as a Predictive Biomarker for Androgen Receptor Axis-Targeted Therapy in De Novo Metastatic Castration-Sensitive Prostate Cancer. Clin Cancer Res. 2022 Nov 14;28(22):4917-4925. doi: 10.1158/1078-0432.CCR-22-2228. PMID: 36088616.
- Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, Cieslik M, Benelli M, Robinson D, Van Allen EM, Sboner A, Fedrizzi T, Mosquera JM, Robinson BD, De Sarkar N, Kunju LP, Tomlins S, Wu YM, Nava Rodrigues D, Loda M, Gopalan A, Reuter VE, Pritchard CC, Mateo J, Bianchini D, Miranda S, Carreira S, Rescigno P, Filipenko J, Vinson J, Montgomery RB, Beltran H, Heath EI, Scher HI, Kantoff PW, Taplin ME, Schultz N, deBono JS, Demichelis F, Nelson PS, Rubin MA, Chinnaiyan AM, Sawyers CL. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A. 2019 Jun 4;116(23):11428-11436. doi: 10.1073/pnas.1902651116. Epub 2019 May 6. PMID: 31061129; PMCID: PMC6561293.
- Corn PG, Heath EI, Zurita A, Ramesh N, Xiao L, Sei E, Li-Ning-Tapia E, Tu SM, Subudhi SK, Wang J, Wang X, Efstathiou E, Thompson TC, Troncoso P, Navin N, Logothetis CJ, Aparicio AM. Cabazitaxel plus carboplatin for the treatment of men with metastatic castration-resistant prostate cancers: a randomised, open-label, phase 1-2 trial. Lancet Oncol. 2019 Oct;20(10):1432-1443. doi: 10.1016/S1470-2045(19)30408-5. Epub 2019 Sep 9. Erratum in: Lancet Oncol. 2020 Jan;21(1):e14. PMID: 31515154; PMCID: PMC6858999.
- Zang PD, Chawla NS, Barragan-Carrillo R, Chehrazi-Raffle A, Tripathi A, Pal SK, Dorff TB. Tumor Mutational Burden in Metastatic Castration-Resistant Prostate Cancer and Response to Checkpoint Inhibition. JAMA Oncol. 2024 Apr 1;10(4):531-532. doi: 10.1001/jamaoncol.2023.6817. PMID: 38329743; PMCID: PMC10853862.
- Lincoln SE, Nussbaum RL, Kurian AW, Nielsen SM, Das K, Michalski S, Yang S, Ngo N, Blanco A, Esplin ED. Yield and Utility of Germline Testing Following Tumor Sequencing in Patients With Cancer. JAMA Netw Open. 2020 Oct 1;3(10):e2019452. doi: 10.1001/jamanetworkopen.2020.19452. Erratum in: JAMA Netw Open. 2021 Jul 1;4(7):e2123147. PMID: 33026450; PMCID: PMC7542302.


