(UroToday.com) In an oral abstract presentation on the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022 focussed on prostate cancer, Dr. Srinivas provided a discussion following presentations from Dr. Crabb and Dr. Rosenberg on the ATLANTIS and BAYOU trials, respectively.
Contextualizing these studies, Dr. Srinivas considered the question of where we stand with PARP inhibitors in urothelial cancer. In considering this question, she emphasized the current treatment paradigm in advanced urothelial cancer. Across three lines of therapy, there are many treatment modalities including cytotoxic chemotherapy, immune checkpoint inhibitors, targeted therapies, and antibody-drug conjugates. However, to date, there has not been an established role for PARP inhibitors in this disease space.
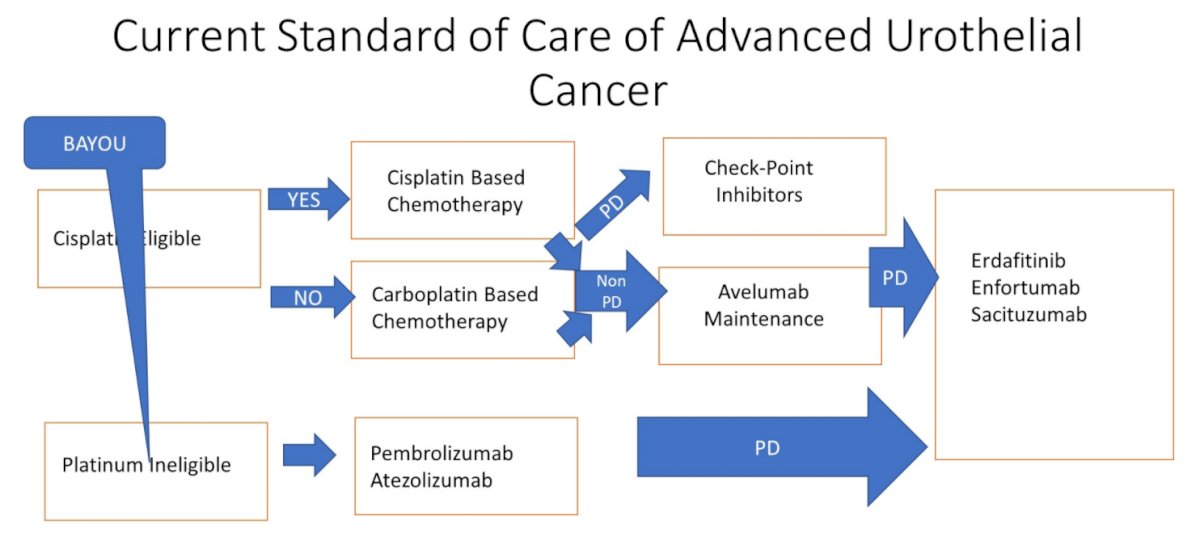
Dr. Srinivas then highlighted the data from BAYOU presented by Dr. Rosenberg. To put these data in context, she highlighted prior data from ATLAS (examining rucaparib monotherapy among patients who had receive two prior lines of therapy) and BISCAY (one of six arm which examined olaparib in combination with durvaluamb in patients who had previously received platinum-based chemotherapy) both of which failed to demonstrate a meaningful benefit to the use of PARP inhibitors. KEYNOTE-052 demonstrated an objective response rate of 29% with pembrolizumab for cisplatin-ineligible patients, a finding that was replicated in real world data.
The BAYOU trial assessed the combination of durvalumab and olaparib compared to durvalumab alone in patients who were treatment naïve but ineligible for cisplatin or carboplatin based chemotherapy.

Dr. Srinivas emphasized that a large proportion of patients in the trial (67% among the combination arm and 63% in the durvalumab only arm) had visceral metastasis. She further noted that patients in the combination arm were more likely to have ECOG performance status 2 (44% vs 37%). Notably, homologous recombination repair (HRR) mutations were seen in 22% of patients in the combination arm and 18% of those in the durvaluamb only arm, predominately ATM and BRCA2 mutations.
Dr. Srinivas emphasized that the results of BAYOU should no difference in progression-free or overall survival for the combination approach. She noted that, compared to KEYNOTE-052 and BISCAY, that objective response rates (ORR) seen in BAYOU were lower than may be expected: for example, in KEYNOTE-052, the ORR was 27% for immune checkpoint inhibitor (pembrolizumab) monotherapy whereas it was 18% with durvalumab monotherapy in BAYOU. Similarly, in BISCAY, combination therapy resulted in an ORR of 36% whereas in BAYOU the ORR for a combination approach was 28%.
Citing data from phase 3 trials including JAVELIN 100 and IMVigor 211, she emphasized that the data to support DDR enhancing immunotherapy is unclear. Thus, summarizing BAYOU, she concluded that these data set a benchmark for PFS and OS in platinum ineligible patients though a combination of durvalumab and olaparib did not demonstrate improved outcomes, despite evidence of activity in patients with HRR mutations.

Moving to the presentation of the rucaparib arm of ATLANTIS from Dr. Crabb, Dr. Srinivas highlighted that this directly overlaps with avelumab maintenance therapy, a treatment approach established on the basis of the JAVELIN Bladder 100 trial.

Dr. Srinivas suggested that, for both progression-free survival (hazard ratio 0.53, 80% CI 0.30-0.92, p=0.07) and overall survival (HR 1.22, 80% 0.62-2.38, p=0.35), there is a trend (though not statistically significant) of benefit for rucaparib, compared to placebo. However, based on JAVELIN Bladder 100, avelumab has set the standard in this setting with proven improvements in overall survival. However, comparing the two trials, she noted that overall survival was somewhat longer in the placebo arm of ATLANTIS (18 months) than in JAVELIN Bladder 100 (14 to 17 months).
Dr. Srinivas then highlighted PARP inhibitor approvals, across tumor sites in cancer emphasizing approvals in prostate cancer, ovarian cancer, pancreatic cancer, and breast cancer. In many of these spaces, the approval is for genomically selected patients.

While not approved in urothelial carcinoma, there are numerous disease spaces in which PARP inhibitors are being assessed including in the neoadjuvant space, in first line systemic therapy for advanced disease, as maintenance therapy, and as subsequent lines of therapy.

Summarizing the question of PARP inhibitors in UC, she emphasized that there are more questions than answers including the lack of data for monotherapy, the number of patients needed to screen to identify eligible candidates, the question of combination or monotherapy, the best setting for use of a PARP inhibitor, the sequencing of therapy, and the role of genomic testing.
Presented by: Sandy Srinivas, MD, Stanford University Medical Center


