(UroToday.com) Dr. Narayan began his discussion with a case of a 72-year-old man who presents with de novo high volume metastatic prostate cancer, symptomatic with vertebral bone pain. His PSA is 320 ng/mL and a prostate needle biopsy showed Gleason grade group 4 disease. He initiates therapy with testosterone suppression and abiraterone and has a good response. Unfortunately, 18 months later, he has rising PSA, new bone lesions, and new bony pain. He undergoes first-line treatment for metastatic castration-resistant prostate cancer (mCRPC) with docetaxel.
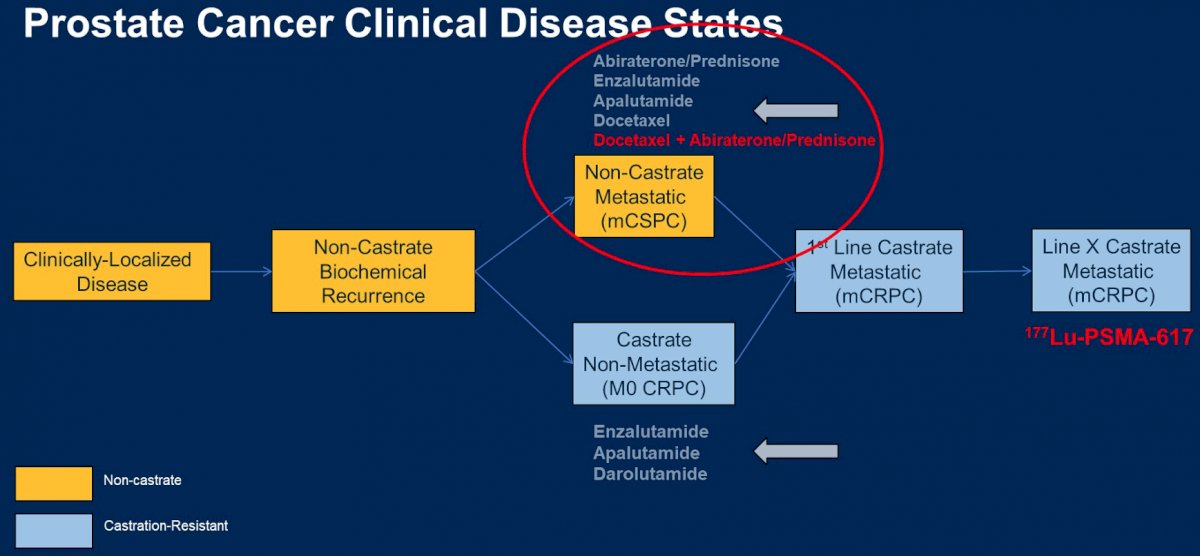
To put this patient case in context, he provided the framework shown below of disease progression from clinically localized disease through lethal castration resistance. He noted that over the past several years, we have seen a shift in treatment approach marked by using effective therapies earlier in the disease course. What then are emerging options for subsequent treatment in mCRPC?

To highlight newer therapies in the more advanced disease state, Dr. Narayan described the VISION trial1 of the beta-emitting radiopharmaceutical lutetium-177 in combination with an antibody against PSMA, a protein expressed on the surface of prostate cancer cells amongst other cell types. This trial included patients with mCRPC who had received treatment previously with both an androgen pathway inhibitor and a taxane chemotherapy, and randomized patients 2:1 to either lutetium or standard of care, the latter which was pre-specified prior to randomization and could not include chemotherapy, immunotherapy, radium-223 or other investigational drug. Importantly, patients underwent a centrally reviewed PSMA-PET scan, and for enrollment were required to have at least one PSMA-positive metastatic lesion and no PSMA-negative metastatic lesions (solid organ met >= 1.0 cm, lymph node >= 2.5 cm, or bony met with soft tissue component >= 1.0 cm). 87% of patients scanned for eligibility met these inclusion criteria.

About 40% of patients had received two androgen pathway inhibitors, and 40% of patients had received two taxane chemotherapies.
Both alternate primary endpoints of imaging-based progression free survival and overall survival favored lutetium PSMA therapy. The median imaging based PFS and median overall survival for the lutetium treatment arm were 8.7 months and 15.3 months, respectively, compared to 3.4 and 11.3 months in the standard of care arm. This difference translates into a 0.62 hazard ratio for death with lutetium therapy relative to standard of care. Key secondary endpoints such as time to first symptomatic skeletal event, overall RECIST response, PSA response and health related quality of life also favored lutetium therapy. The major toxicities of lutetium therapy included fatigue, xerostomia, and cytopenias.
The therapeutic efficacy of lutetium-PSMA was also tested in the randomized phase II TheraP trial2, which randomized patients 1:1 to either lutetium-PSMA or cabazitaxel, a comparison earlier in disease course than that of VISION. Patients with mCRPC who had received docetaxel and were eligible for additional taxane, had progressive disease by PSA and good performance status were eligible. Importantly, this trial performed both FDG-PET and PSMA-PET and excluded patients with either PSMA-low or FDG-positive and PSMA-discordant disease sites. 28% of patients screened were found to have either PSMA-low or discordant disease. The endpoint of this trial was PSA50 response, which occurred more commonly in lutetium-PSMA treated patients (66%) compared to cabazitaxel treated patients (37%). Additionally, lutetium treated patients had fewer grade 3 or 4 adverse events than patients treated with cabazitaxel.
Dr. Narayan concluded his lutetium-PSMA discussion by raising several critical questions that remain to be answered with this treatment modality:1 What is the optimal imaging methodology to choose patients for this therapy,2can it be moved earlier or in combination with other treatments in the disease course of prostate cancer, and3 how will the significant resource and logistics requirements impact implementation of this modality?
He then had a discussion of two distinct treatment agents in the metastatic castration sensitive setting, androgen pathway inhibition versus chemotherapy, specifically focusing on abiraterone versus docetaxel. He referenced a study3 that indirectly compared abiraterone and docetaxel treatment, which noted different patterns and timing of treatment failure, different patterns of toxicity, but no clear difference in overall survival in the examined population. A two-year global quality of life study suggested that global-QOL scores were higher for abiraterone relative to docetaxel, but this did not meet the pre-specific threshold for clinically meaningful difference.4

Finally, Dr. Narayan discussed the recently presented PEACE-1 data, exploring the role of triplet therapy in the metastatic castration sensitive disease context. In this study, patients with de novo mCSPC who had been receiving testosterone suppression for less than three months were randomized to multiple treatment groups. In data presented at ESMO 2021, triplet combination with testosterone suppression, abiraterone, and docetaxel resulted in a 61 month median overall survival, providing a hazard ratio of 0.75 for death relative to testosterone suppression and docetaxel alone.
He concluded by modifying his original disease state slide to include triplet therapy for mCSPC, and lutetium-PSMA in mCRPC.
He then showed a figure from a recent publication5 suggesting a potential decision-making tree for selecting patient treatment in mCSPC, stratifying patients by disease volume.

Presented By: Vivek Narayan, MS, MD, medical oncologist at the University of Pennsylvania
Written By: Alok K. Tewari, MD, PhD, medical oncologist at Dana-Farber Cancer Institute, @aloktewar on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022
References:
1. doi: 10.1056/NEJMoa2107322
2. doi: 10.1016/S0140-6736(21)00237-3
3. doi: 10.1093/annonc/mdy072
4. doi: 10.1200/JCO.21.00728
5. doi: 10.1200/JCO.21.02530


