(UroToday.com) The 2024 GU ASCO annual meeting featured a urothelial carcinoma session and a presentation by Dr. Roger Li discussing urine-based testing for patient selection and genomic characterization of patients with FGFR alteration-positive non–muscle-invasive bladder cancer (NMIBC) treated with TAR-210. Erdafitinib, on oral selective pan-FGFR tyrosine kinase inhibitor, is approved for locally advanced or metastatic urothelial carcinoma in adults with susceptible FGFR3/2 alterations after progression on platinum-containing therapy.1-2
TAR-210 is an intravesical drug delivery system that is designed to provide local, continuous release of erdafitinib. Its safety and efficacy are being evaluated in a first-in-human clinical study of patients with bladder cancer whose tumors harbor select FGFR alterations: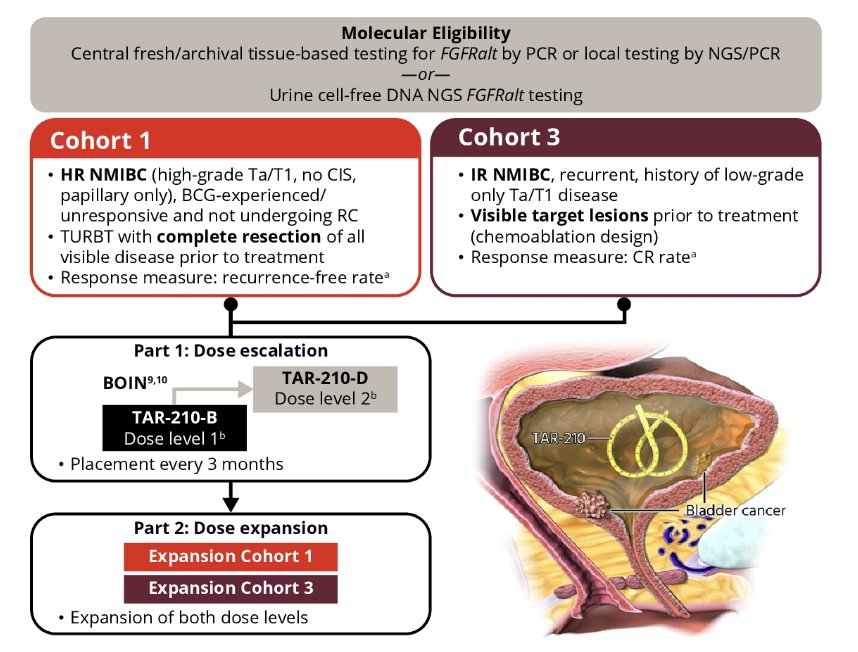
Dr. Li notes that to overcome tissue-based challenges in identifying susceptible FGFR alterations to select patients for treatment with TAR-210, Janssen partnered with Predicine to use a proprietary urine cell-free DNA diagnostic assay called PredicineCARE. Presented at GU ASCO 2023, validation of the urine assay to detect FGFR alterations was previously demonstrated using urine samples collected in a highly controlled manner via a collaboration with Stratifyer Molecular Pathology GmbH. However, evaluation of the urine assay for diagnostic screening in a real-world setting is currently lacking. At GU ASCO 2024, Dr. Li reports preliminary results of the urine assay for patient selection, as well as on the characterization of the urine-defined genomic landscape in screened patients.
Enrollment was based on detection of prespecified FGFR alterations from either tumor tissue obtained from previous biopsies (Qiagen Therascreen FGFR assay) or urine samples obtained prior to enrollment (PredicineCARE next-generation sequencing assay). As of June 20, 2023, urine assay performance was compared to the tissue test from all screened patients with NMIBC (n = 178):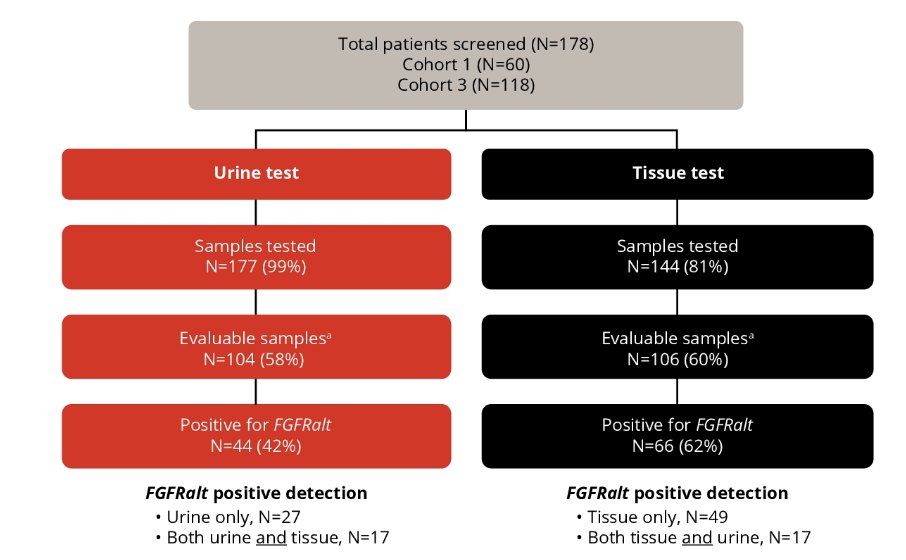
The proportions of samples that yielded results were 60% and 58% from tissue and urine, respectively, while the FGFR alterations detection rates in the subsets that yielded results were 62% and 42%, respectively. FGFR3 S249C was the most frequent alteration detected in both tissue (48%) and urine (61%):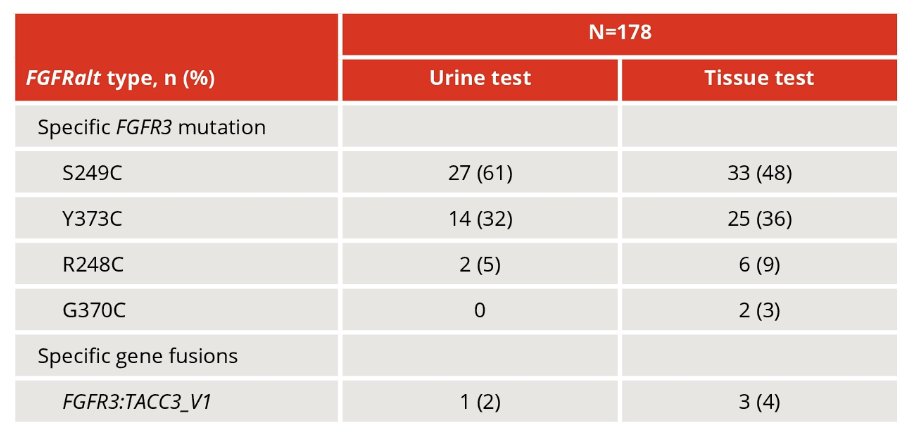
For 36% of urine samples in which FGFR alterations were detected, there was no corresponding tissue result. Of the disease-evaluable patients with high-risk NMIBC (n = 11) or intermediate-risk NMIBC (n = 15), 18.2% and 33%, respectively, were enrolled based on urine assay alone due to insufficient tissue samples. A recurrence-free rate of 82% and a complete response rate of 87% were achieved at the first disease evaluation amongst patients with high-risk NMIBC and intermediate risk NMIBC, respectively: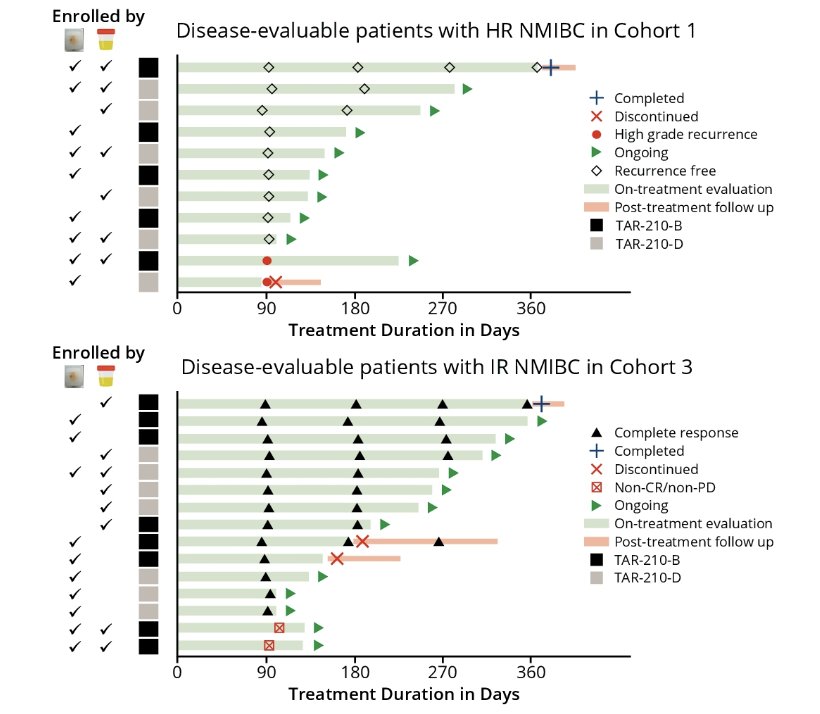
All patients (high-risk NMIBC, n = 2; intermediate risk NMIBC, n = 5) enrolled by “urine only” were recurrence-free or achieved a complete response. Based on the PredicineCARE panel, comprehensive genomic assessment of urine samples from all screened patients with NMIBC was performed: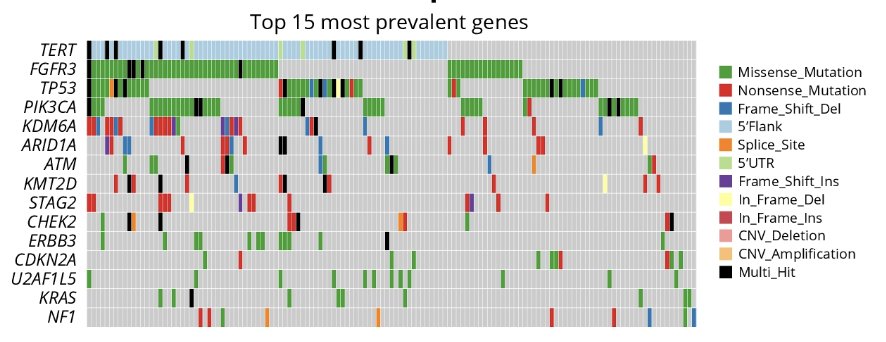
Finally, the prevalence of alterations detected was similar to that described in prior studies using tissue-based testing.
Dr. Li concluded his presentation discussing urine-based testing for patient selection and genomic characterization of patients with FGFR alteration-positive NMIBC treated with TAR-210 with the following take-home points:
- Implementing a urine-based assay expands the molecular testing methods to identify (27%) additional patients that may respond to TAR-210
- The spectrum of genomic alterations detected using the urine test was similar to that described in prior studies using tissue-based testing
- All patients in Cohort 3 enrolled by urine test showed clinical activity
- These data highlight that the complex genomic landscape of bladder cancer can be assessed from urine
- Data from this study support further clinical evaluation of the urine test
Presented by: Roger Li, MD, Department of Genitourinary Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
Related content: ASCO GU 2024: Urine-Based Testing for Patient Selection and Genomic Characterization of Patients with FGFR Alteration-Positive NMIBC Treated with TAR-210
References:
- Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2019 Jul 25;381(4):338-348.
- Loriot Y, Matsubara N, Park SH, et al. Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2023 Nov 23;389(21):1961-1971.


