(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a renal and rare tumors oral abstract session. Dr. Ulka Vaishampayan delivered the discussant for the preceding two presentations:
- Subcutaneous nivolumab (NIVO SC) versus intravenous nivolumab in patients with previously treated advanced or metastatic clear cell renal cell carcinoma: Pharmacokinetics, efficacy, and safety results from CheckMate 67T
- Belzutifan versus everolimus in participants with previously treated advanced renal cell carcinoma: Patient-reported outcomes in the phase 3 LITESPARK-005 study
CheckMate 67T (NCT04810078) is a multicenter, randomized, open-label, non-inferiority phase 3 study that evaluated the pharmacokinetics and objective response rate (ORR) of NIVO SC versus IV in immunotherapy-naïve patients with locally advanced or metastatic ccRCC treated in the 2nd or 3rd line settings. Why evaluate a subcutaneous administration approach? There are pros and cons to IV versus subcutaneous approaches, as summarized below, with the main advantage of a subcutaneous approach being the ease of administration and the ability to be done either at home or in the clinic, which saves time for patients and staff. This potentially reduced health care resources utilization would have been of utmost importance during the recent COVID-19 pandemic. An additional rationale for evaluating the subcutaneous versus intravenous approach for nivolumab is its wide therapeutic index, with previous data demonstrating similar efficacy and safety for nivolumab across a wide range of administered dosages.1

In CheckMate 67T, patients underwent 1:1 randomization to either NIVO SC 1,200 mg + rHuPH20 every 4 weeks (n=248) or NIVO 3mg/kg every 2 weeks (n=247). Treatment was continued until disease progression, unacceptable toxicity, consent withdrawal, completion of two years of treatment, or death. The vast majority (~90%) had received one prior line of therapy. Approximately 82% had undergone a prior nephrectomy. Non-inferiority for the co-primary pharmacokinetic endpoints was met, with SC nivolumab + rHuPH20 (hyaluronidase allowing for rapid, improved absorption) actually showing higher exposure levels to nivolumab.
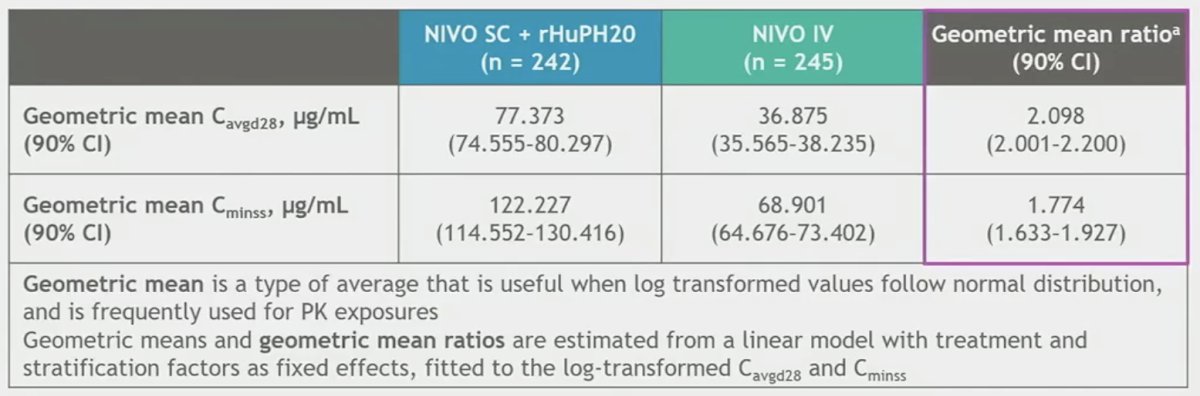
Similarly, non-inferiority for the key powered secondary endpoint ORR by BICR was met, with an ORR of 60% in the subcutaneous arm versus 45% in the intravenous arm (RR: 1.33, 95% CI: 0.94 – 1.87). While the median PFS was longer with NIVO SC (7.2 versus 5.7 months), this difference did not meet statistical significance (HR: 1.06, 95% CI: 0.84 – 1.34).

Dr. Vaishampayan’s takeaways from this study were as follows:
- Positives
- Subcutaneous nivolumab +rHuPH20 showed increased exposure to nivolumab compared to the IV route, per pharmacokinetic profile
- Efficacy in terms of response rate was improved (ORR: 60% with SC vs 45% with IV)
- PFS and time to response were not changed (HR=1.05)
- Non-inferiority in terms of drug exposure and pharmacokinetic profile is proven by this study.
- It is feasible to substitute SC nivolumab where IV single agent nivolumab is currently used
- Concerns
- Anti-drug antibody rate was higher with SC (22.5%) versus 7% with IV, which ultimately could lead to increased resistance to nivolumab.
- Nivolumab in RCC resulted in an OS benefit, not PFS, and the study was not powered to show this and will need prolonged follow up to reveal that.
- In RCC, nivolumab single agent use is less common, usually used in combination with other agents. How will the combinations with ipilimumab or cabozantinib affect nivolumab drug exposure with SC administration?
Dr. Vaishampayan concluded that SC nivolumab is likely comparable to IV nivolumab and has the potential to make therapy more convenient and easier tolerated.
Next, Dr. Vaishampayan moved on to discuss the PROs from LITESPARK-005, presented earlier by Dr. Powles. She noted the importance of patient reported outcomes, as opposed to physician reported outcomes. It has been consistently (and convincingly) been demonstrated that physicians under-report symptoms that matter to patients, often by as much as 50%.

The randomized, phase 3 LITESPARK-005 study showed significant improvement in PFS and ORR with belzutifan versus everolimus in patients with advanced clear cell RCC after prior immune checkpoint and anti-angiogenic therapies, prolonging the primary endpoint of PFS (HR: 0.75, 95% CI: 0.63 – 0.90, p<0.001) and improving ORR (key secondary end point), with an estimated difference of 18.4% (p<0.001).2

In this report of PROs from LITESPARK-005, formal comparisons were performed between PRO scores at baseline and week 17 (interim outcomes were reported, but not formally compared). Notably, 10% more patients completed questionnaires at week 17 in the belzutifan arm, as compared to everolimus, which may reflect improved treatment responses (i.e., patients with improved responses and potentially better symptoms may be more motivated to complete the questionnaires). This analysis of PROs demonstrated:
- Better disease-specific symptoms and quality of life with belzutifan compared with everolimus in patients with advanced clear cell RCC
- Belzutifan was associated with meaningfully longer time to deterioration in the disease-specific FKSI-DRS and global EORTC QLQ-C30 GHS/QoL scales, compared with everolimus
- Overall, least squares and empirical mean changes from baseline in FKSI-DRS and EORTC QLQ-C30 suggested greater worsening in the everolimus group versus stability in the belzutifan group
Dr. Vaishampayan noted that although 8% more patients in the belzutifan arm had quality of life improvements, almost half of patients in both arms had stable disease, and only 5% more patients had quality of life deterioration on everolimus, as compared to belzutifan.

And while belzutifan outperformed everolimus overall, both groups had decreased QLQ-C30 scores from baseline. Is this related to disease or toxicity?

Her takeaways from the LITESPARK-005 PROs report were as follows:
- Positives
- Conducting a PROs analysis in a cohort of pre-treated RCC patients is extremely challenging and should be commended, particularly as many such patients are often managed with palliative intent
- The mean kidney-specific FKSI scores were higher with belzutifan treated patients
- Time to deterioration was prolonged in the belzutifan arm as compared to everolimus
- In addition to ORR and PFS, belzutifan showed modest benefit in QoL, which was yet another parameter of success for belzutifan over everolimus
- Concerns
- Impossible to separate the drug-related toxicities from cancer-related deterioration
- QOL changes may not correlate with clinical efficacy
- Severe worsening of QoL is typically not captured, as hospitalized patients cannot complete PRO
- Should only the patients who completed both baseline and post therapy time point (week 17) be included in analysis so a true assessment of the effect of the drug is determined?
Presented by: Ulka N. Vaishampayan, MBBS, Professor, Department of Internal Medicine, Division of Hematology and Oncology, University of Michigan Ann Arbor, MI
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
References:1. George S, Motzer RJ, Hammers HJ, et al. Safety and Efficacy of Nivolumab in Patients with Metastatic Renal Cell Carcinoma Treated Beyond Progression. JAMA Oncol. 2016;2(9):1179-86.
Related Content: ASCO GU 2024: Subcutaneous Nivolumab Versus Intravenous Nivolumab in Patients With Previously Treated Advanced or Metastatic Clear Cell Renal Cell Carcinoma: Pharmacokinetics, Efficacy, and Safety Results From CheckMate 67T
ASCO GU 2024: Belzutifan Versus Everolimus in Participants With Previously Treated Advanced Renal Cell Carcinoma: Patient-Reported Outcomes in the Phase 3 LITESPARK-005 Study


