(UroToday.com) The 2024 American Urological Association (AUA) annual meeting featured a session on bladder cancer trials in progress, and a presentation by Dr. Sandeep Reddy discussing QUILT 2.005, a trial comparing intravesical BCG in combination with the IL-15 superagonist N-803 to BCG alone in patients with BCG-naïve non muscle invasive bladder cancer. Standard therapy for newly diagnosed high risk non muscle invasive bladder cancer is intravesical instillation of BCG typically following the SWOG protocol. Although BCG is effective, cancer will recur in ~40% of patients. When BCG is delivered in the bladder, the hypothesis is that it provides a stimulus for the establishment for trained immunity memory. However, an efficacious response to BCG is reliant upon sufficient natural killer cells and T-cells. N-803 (nogapendekin alfa inbakicept: ANKTIVA®), is an interleukin-15 superagonist (IL-15), that promotes activation and proliferation of natural killer cells, CD8+ T cells and memory T cells without expanding immunosuppressive T-reg cells:
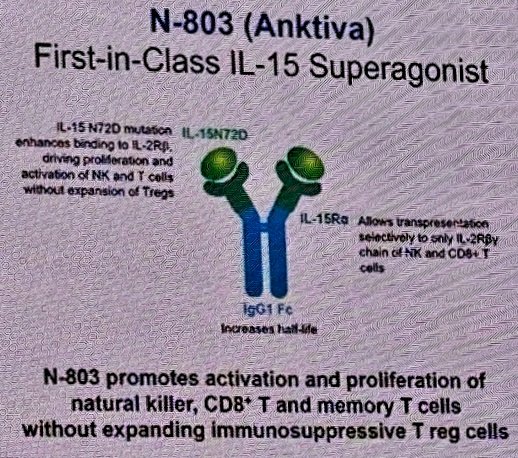
N-803 synergizes with BCG to elicit durable complete responses and has recently been FDA-approved for BCG-unresponsive NMIBC CIS, with or without papillary tumors1:
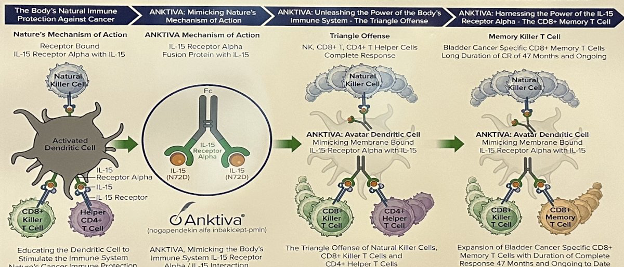
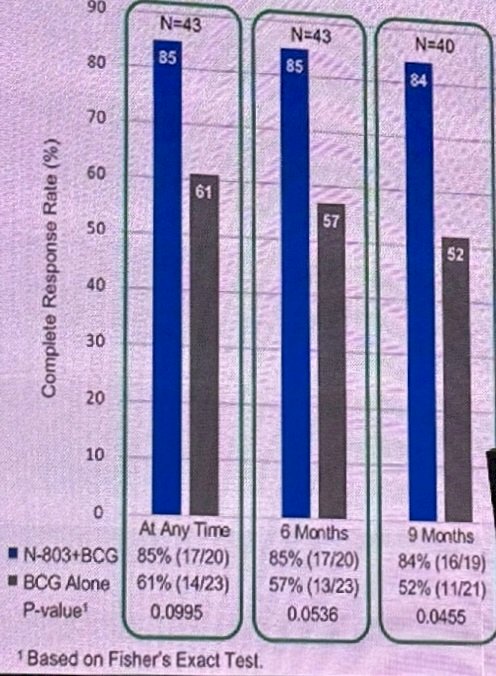
In phase 1, there was durable complete remission among 9 patients with CIS and papillary non-muscle invasive bladder cancer treated with BCG + N-803 (ANKTIVA®). As requested by the Agency, there was an unplanned interim analysis of the efficacy results in CIS for the phase 2 QUILT-2.005 trial, which showed an improvement in complete response rate over time and contribution of effect of N-803 inducing memory T-cells:
The trial design for phase IIb QUILT-2.005 is for participants to be randomized 1:1 to one of two independent treatment arms based on disease and ECOG status. Participants receive either intravesical 400 ug N-803/instillation + 50 mg BCG or BCG alone weekly for six consecutive weeks, followed by maintenance treatment for 3 consecutive weeks at 3, 6, 12, and 18 months. At 3 months, re-induction with another 6 weeks of therapy for eligible patients is an option. Cohort A of QUILT-2.005 will have CIS +/- Ta or T1 disease patients, and Cohort B will have high grade papillary Ta or 1 disease patients.
Participants must be >= 18 years of age, with ECOG performance status 0, 1, or 2. They must also have histologically confirmed CIS +/- Ta/T1 disease (cohort A) or high grade papillary Ta/T1 only disease (Cohort B), with diagnostic biopsy within 3 months of treatment start and a cystoscopy demonstrating no resectable disease within 6 weeks. Participants must be BCG-naïve (or last BCG > 3 years prior) and have not received more than a single post-operative dose of mitomycin C or gemcitabine following the most recent screening TURBT/biopsy.
The primary outcomes are complete response rate and disease free survival with the time frames of 12 and 24 months, respectively. The complete response rate for Cohort A and disease free survival for Cohort B will be compared between treatment arms using cystoscopy, confirmatory biopsy, and urine cytology. Safety will be assessed by number/severity of treatment related adverse events over 48 months.
The first phase IIb patient was enrolled on October 15, 2015. Enrollment was limited as a result of BCG shortage, as the sponsor is providing TICE BCG in the trial and prioritization to QUILT 3.3032 BCG unresponsive pivotal trial. As of April 2024, cohort A had enrolled 121 of 366 planned participants, and cohort B had enrolled 74 of 230 planned participants. Currently, participants are being actively enrolled in the phase IIb of QUILT 2.005.
Presented by: Sandeep Reddy, MD, ImmunityBio, San Diego, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Urological Association (AUA) Annual Meeting, San Antonio, TX, Fri, May 3 – Mon, May 6, 2024.
References:
- Chamie K, Chang SS, Kramolowsky E, et al. IL-15 Superagonist NAI in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer. NEJM Evid 2022; 2(1)


