(UroToday.com) Following the presentation by Dr. Karim Fizazi on the phase 3 PEACE-1 trial. Dr. Axel Heidenreich provides a discussion presentation on important considerations for interpretation of this trial. The PEACE-1 phase 3 trial studied abiraterone acetate plus prednisone added to androgen deprivation therapy and docetaxel in men with de novo metastatic castration-sensitive prostate cancer (mCSPC).
His first critique of the presentation is something Dr. Fizazi himself alluded to in the initial talk. The standard of care (SOC) for de novo metastatic castration-sensitive prostate cancer (mCSPC) has rapidly changed over the past decade, even as the PEACE-1 trial was accruing.

As seen above, ADT monotherapy had been the longstanding SOC and, in fact, was the initial SOC for the PEACE-1 trial. The addition of docetaxel, based on CHAARTED and STAMPEDE trial data, was incorporated later into the study as part of the SOC – but, Dr. Heidenreich notes that at this time, even that has become outdated. Indeed, due to data suggesting ADT+docetaxel is best suited for higher volume metastatic burden, maybe only ~15% of patients with de novo CSPC would be getting ADT+docetaxel as SOC in the real world at this time – most patients would be received ADT with an oral agent such as enzalutamide, apalutamide or abiraterone acetate+prednisone (AAP) itself.
His next point was to focus on the fact that the study only included men with de novo mCSPC – and not men who had prior primary therapy of their prostate cancer. Based on analysis by Kyriakopoulos et al.1, using the CHAARTED dataset, men with de novo disease appear to have worse outcomes compared to men with prior local therapy.
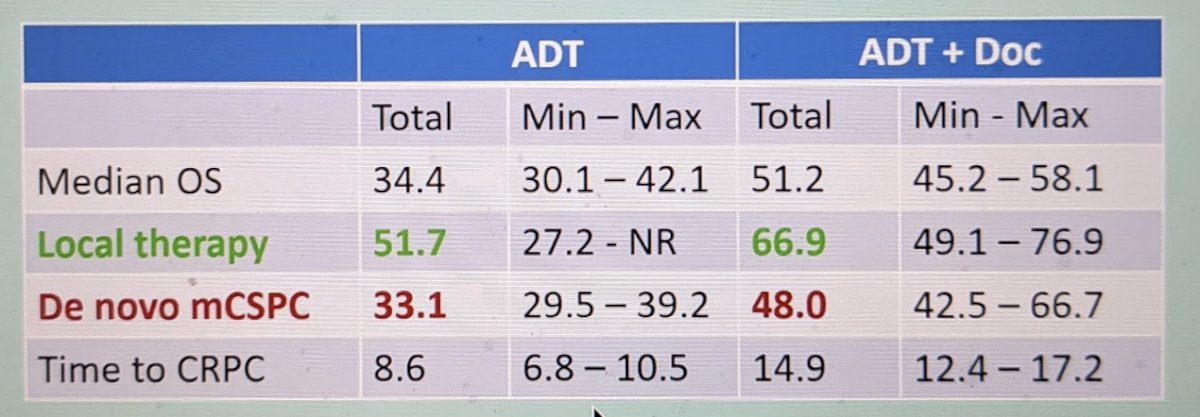
Hence, this should be accounted for when interpreting the PEACE-1 data.
When compared to patients from the ADT+docetaxel studies (CHAARTED and STAMPEDE), he also noted that men in the PEACE-1 trial had slightly more patients with lower volume metastatic burden and lower PSA levels at entry – which may shift the results in favor of better long-term outcomes compared to the original studies.
His next main critique, which is not unexpected, is that while the PEACE-1 study demonstrated significant rPFS benefit, the overall survival (OS) data is still immature and lacking. He naturally questions whether practice standars should change based on rPFS data alone – is this an effective surrogate marker for OS? To address this, he alluded to an abstract as ASCO 2021 by Halabi et al.,2 which identified all phase 3 RCTs for metastatic CRPC between 2004 and 2020 that reported rPFS, PFS or OS. They had a prespecified cutoff to determine if rPFS was a surrogate for OS (R2 0.7 or higher). Overall, they found there was only a moderate correlation between rPFS and OS (R2 = 0.46) – and since it did not meet the cutoff, they concluded that rPFS cannot be used as a surrogate for OS.
His next point focused on the rPFS benefit of essentially triplet therapy (ADT+docetaxel+AAP) compared to doublet therapies already established in this disease space. These are summarized below:

Based on the above, he argues that the added benefit of triplet therapy is minimal considering the addition of two more medications (abiraterone and prednisone).
Ultimately, his take home points from the study are as follows:
1) PEACE-1 does not incorporate more up to date SOC regimens
2) ADT+docetaxel is SOC only for high volume metastatic burden patients now, which account for maybe 15% of de novo CSPC patients
3) Adding AAP to this combination may add long-term toxicity (as seen demonstrated by all of these oral agents (cardiovascular, metabolic))
4) rPFS is NOT a surrogate marker for OS – so we should wait for OS data
5) We have alternative treatment options with equivalent oncologic efficacy
6) Oncologic efficacy of subsequent therapy is unknown – how does this impact the ability to treat patients who recur?
Therefore, he would argue that this is NOT the new standard of care.
Presented by: Axel Heidenreich, Prof. Dr. med., Professor of Urology and Chairman and Director of the Department of Urology, University Hospital, RWTH Aachen, Aachen, Germany
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Assistant Professor of Urology, Sidney Kimmel Cancer Center, Thomas Jefferson University, @tchandra_uromd on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
1. Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol. 2018 Apr 10;36(11):1080-1087. doi: 10.1200/JCO.2017.75.3657.
2. Halabi, S. (2021). ASCO 2021: Radiographic Progression-Free Survival as a Surrogate Endpoint of Overall Survival in Men with Metastatic Castrate-Resistant Prostate Cancer. Retrieved 9 July 2021, from https://www.urotoday.com/conference-highlights/asco-2021/asco-2021-prostate-cancer/130091-asco-2021-radiographic-progression-free-survival-as-a-surrogate-endpoint-of-overall-survival-in-men-with-metastatic-castrate-resistant-prostate-cancer.html


