(UroToday.com) Dr. Karim Fizazi presents the results of the PEACE-1 randomized phase 3 clinical trial with a focus on the impact of adding abiraterone acetate + prednisone to ADT+docetaxel on radiographic progression-free survival (rPFS) during this session at the 2021 European Association of Urology, (EAU) annual meeting. The results of the PEACE-1 randomized phase 3 clinical trial were previously presented at ASCO 2021.
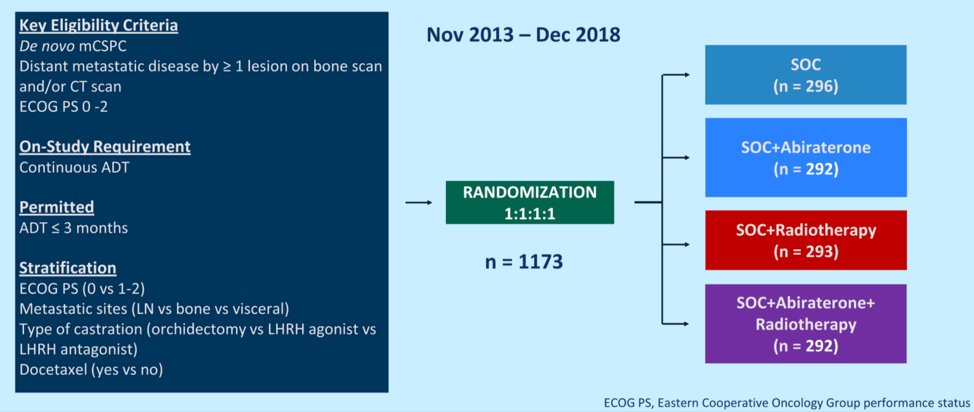
The PEACE consortium (Prostate Cancer Consortium in Europe) is an academic European program that aims to conduct phase 3 trials for men with prostate cancer. The PEACE-1 trial was sponsored by Unicancer, and 7 countries contributed to its accrual. Candidates for this trial had de novo metastatic castration-sensitive prostate cancer (mCSPC) and could have received up to 3 months of ADT prior to randomization. Patients were stratified by metastatic site, performance status, type of testosterone lowering therapy, and the use of docetaxel. A total of 1173 men were randomized 1:1:1:1 as shown below. Standardized treatments included continuous ADT or bilateral orchiectomy, with or without docetaxel at 75 mg/m2 every three weeks for six cycles. Abiraterone treatment consisted of 1000 mg/day with prednisone 5 mg twice per day until disease progression or intolerance and was administered along with docetaxel for patients who underwent chemotherapy. Radiotherapy to the prostate was delivered in 37 doses to a cumulative dose of 74 Gy after patients completed docetaxel if receiving chemotherapy.
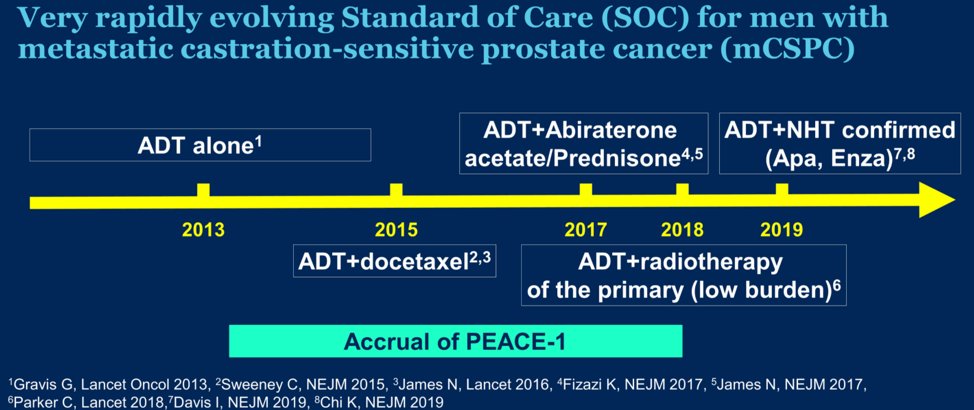
As Dr. Fizazi started off his talk, he immediately alluded to the fact that the standard of care (SOC) changed throughout the accrual period of the study. Various amendments were implemented during the course of the trial due to the evolving standard of care in this disease (see below for timeline in relation to PEACE-1 accrual). After 2015, docetaxel was permitted as part of the standard of care per the investigator’s discretion and patient consent. After LATITUDE and STAMPEDE were reported, it became unethical to administer ADT alone, and so docetaxel administration (without abiraterone) was made mandatory.
As a reminder, for this study, the two co-primary endpoints of the study were radiographic progression-free survival (rPFS) and overall survival. The trial used a 2 x 2 factorial design aimed at answering two questions, the role of abiraterone and the role of radiotherapy on top of the standard of care. The two abiraterone arms (with or without radiotherapy) were pooled for subsequent analysis. Again, for this presentation, the focus is on the impact of adding abiraterone acetate to SOC therapy (which is ADT +/- docetaxel in this study).
Patient characteristics of the study are detailed below:
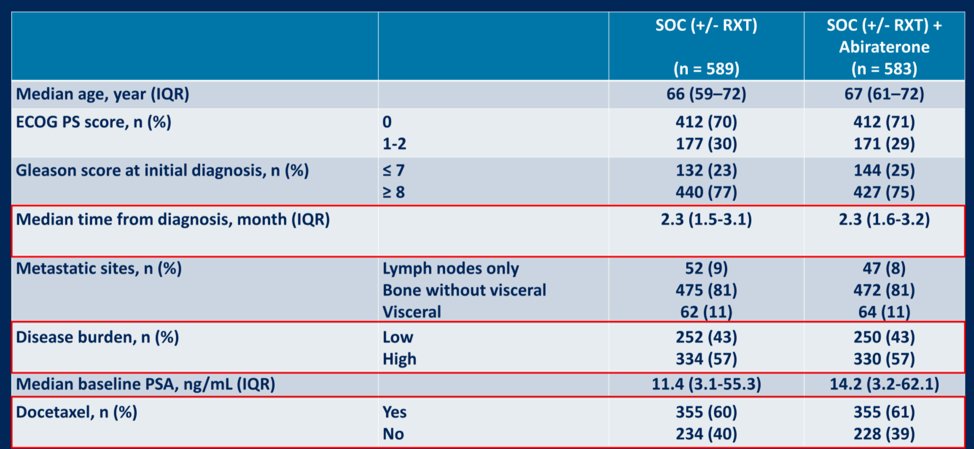
A total of 57% of patients had a high metastatic burden and 60% of patients received docetaxel. The median follow-up for the ADT + docetaxel (n = 355) and the ADT + docetaxel + abiraterone (n = 355) was 36 months. The median time to discontinuation of abiraterone in the abiraterone group was 31.4 months. The median number of docetaxel cycles administered was six.
He then started to go into the details of the radiographic progression free-survival analyses. Again, in this presentation, he specifically compared men who received ADT (LHRH agonist or antagonist) and docetaxel as SOC vs. men who received SOC and abiraterone acetate/prednisone (AAP)
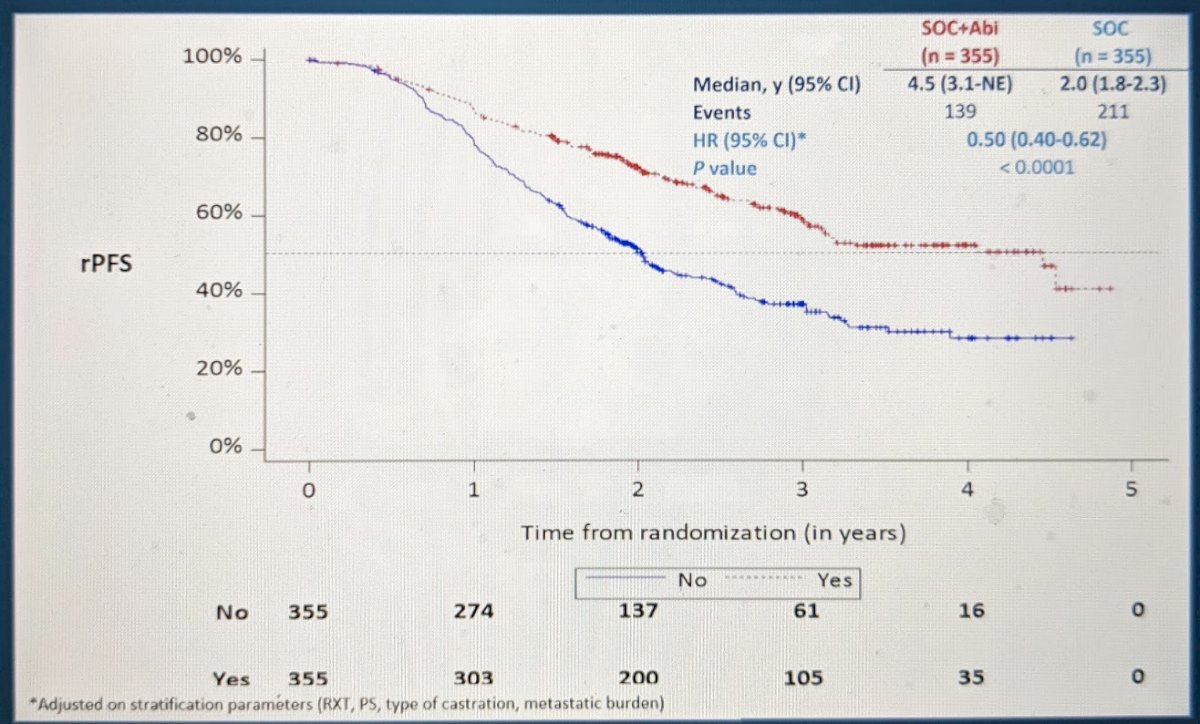
First, men who received the additional AAP had a 2.5 year median rPFS benefit (4.5 vs. 2.0 years, HR 0.50, p <0.0001)!
When broken down into high-volume and low-volume metastatic disease by CHAARTED criteria, the benefit persisted in both cohorts, but was predominantly in the high volume cohort.
In the high volume cohort, men who received the additional AAP had a 2.5 year median rPFS benefit (4.1 vs. 1.6 years, HR 0.47, p <0.0001).
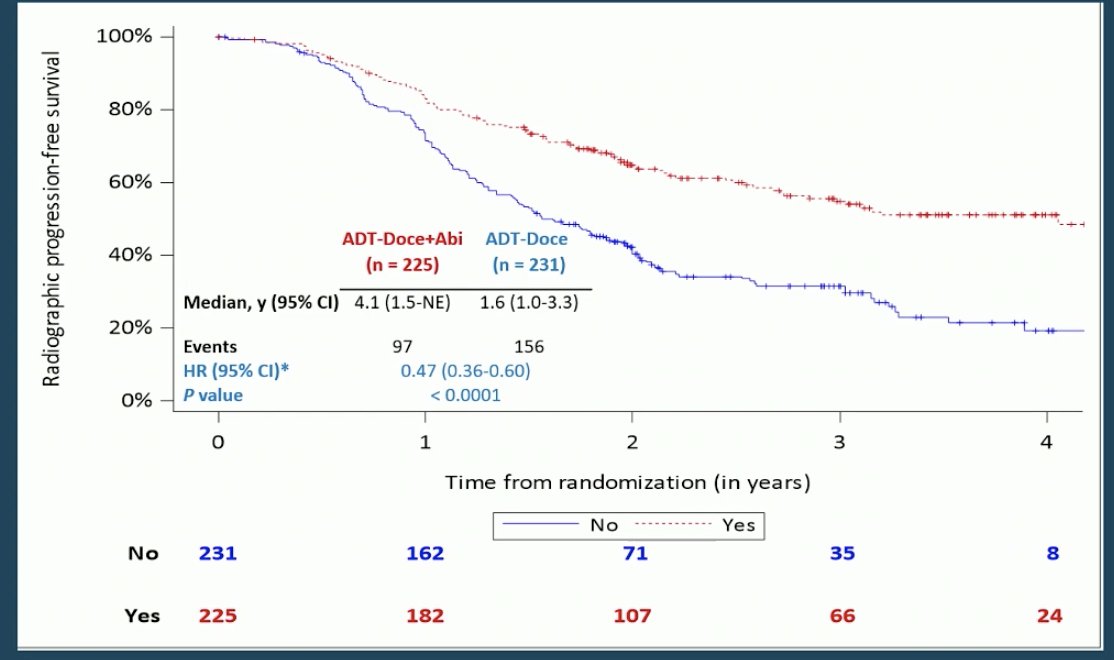
When broken down by type of ADT (LHRH antagonist vs. agonist), there was a similar benefit in both cohorts.
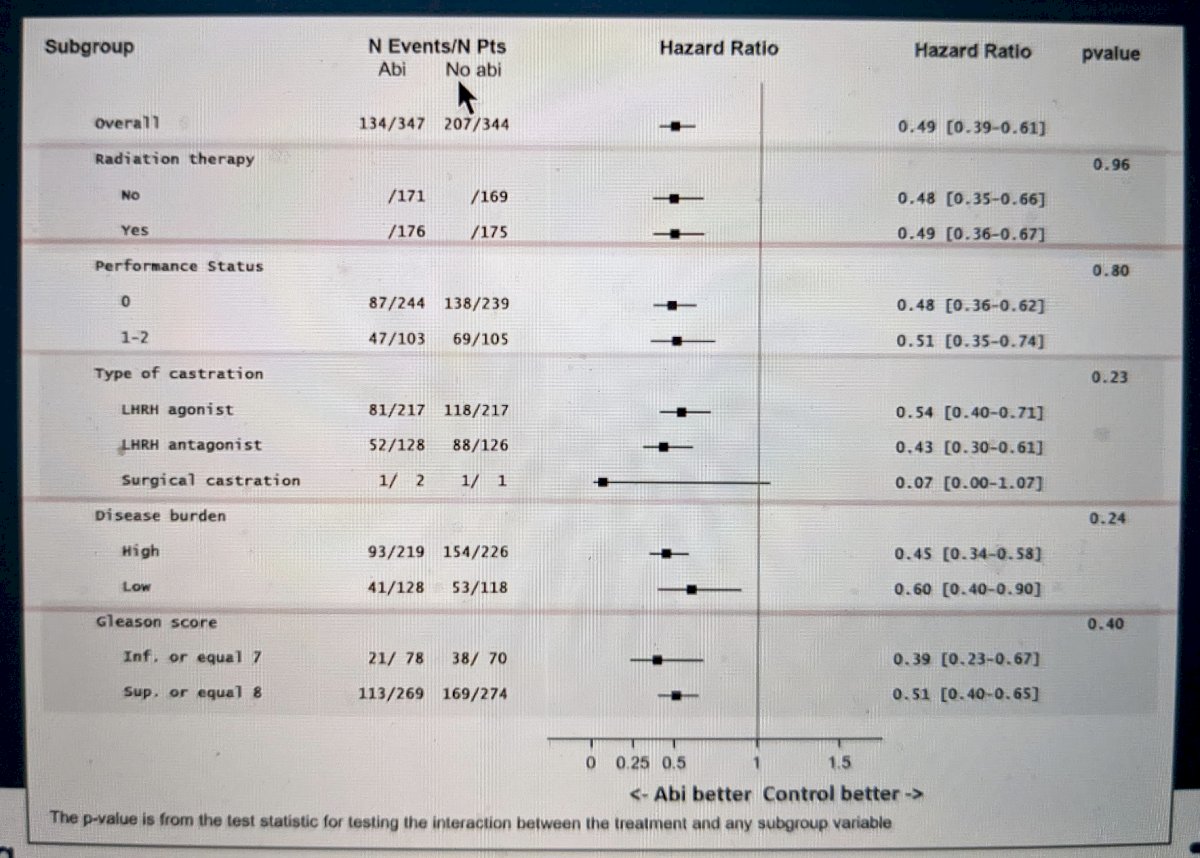
Ultimately, as seen in the forest plot below, regardless of the sub-group analysis, the addition of AAP to ADT+docetaxel appeared to provide significant rPFS benefit.
In the interest of time, Dr. Fizazi did not reiterate toxicitiy data that has been previously presented – as noted in our prior coverage, within the docetaxel group, the rate of grade 3-5 toxicity was relatively similar whether abiraterone was given, with the exception of liver dysfunction and hypertension.
Based on all the above, Dr. Fizazi concludes that:
1) Adding Abiraterone acetate and prednisone to ADT+doxetaxel significantly improves radiographic progression-free survival in men with de novo metastatic prostate cancer
2) The benefit is seen in both high and low volume disease
3) He notes that there’s no meaningful additional short-term toxicity.
4) Overall survival data is still maturing. The study authors hope to present this data later this year.
Despite the lack of overall survival data, he feels the added 2.5 median rRFS benefit alone may warrant utilizing ADT+docetaxel+AAP at the new standard of care for men with de novo metastatic prostate cancer.
Presented by: Karim Fizazi, MD, Ph.D., medical oncologist at Gustave Roussy, and a full professor in Oncology at the University of Paris-Saclay in Villejuif, France.
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Assistant Professor of Urology, Sidney Kimmel Cancer Center, Thomas Jefferson University, @tchandra_uromd on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
Related Content:
Discussion: EAU 2021: PEACE-1 Phase 3 Clinical Trial and Important Considerations For Its Interpretation
First Results of PEACE-1 A Phase 3 Trial with a 2x2 Factorial Design of Abiraterone Acetate plus Prednisone and/or Local Radiotherapy in Men with De Novo Metastatic Castration-Sensitive Prostate Cancer mCSPC - Karim Fizazi


