(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a plenary session on living with advanced kidney cancer and urothelial cancer, and a presentation by Dr. Laurence Albiges discussing that for first line metastatic kidney cancer, we should be using triplet combinations at any cost.
Dr. Albiges notes that the reason we treatment intensify is to address tumor heterogeneity and innate/acquired resistance in oncology. A multidrug approach addresses tumor heterogeneity and reduces the risk of clonal resistance, providing cures for several tumor types, including germ cell tumors and lymphoma: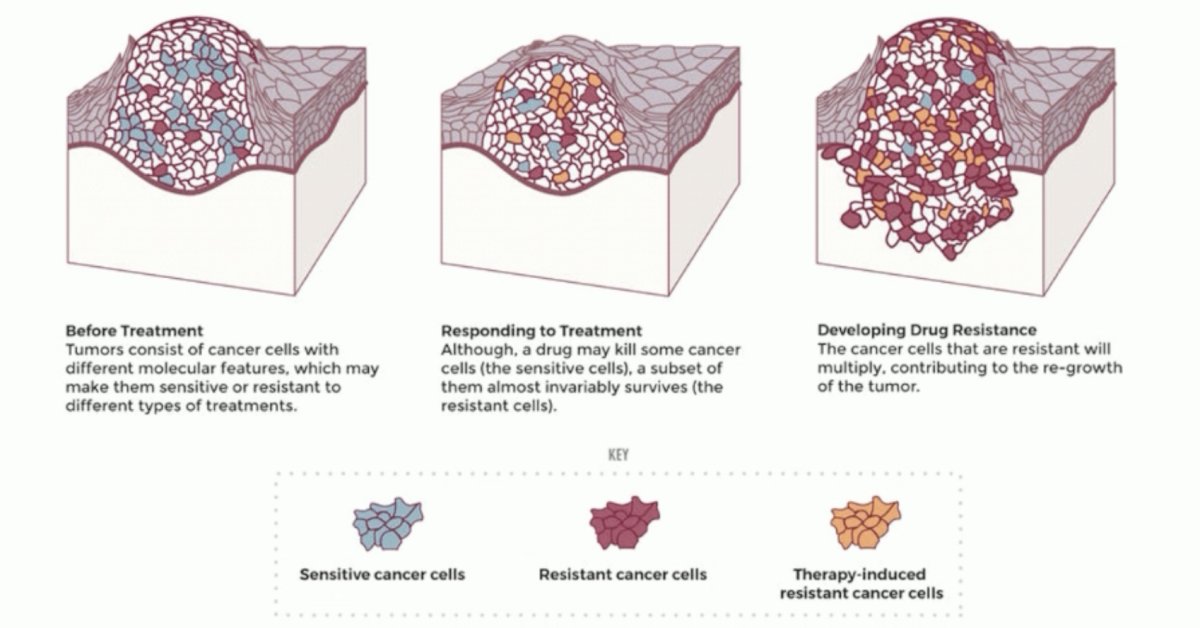
Why does intensification make sense in frontline clear cell RCC? To obtain the best of different approaches, both early and late endpoints. This is likely secondary to several reasons, according to Dr. Albiges. First, the immunotherapy + TKI benefit results in improved short term endpoints, including progression free survival and objective response rate:
Second, there is a long term overall survival benefit for IMDC intermediate and poor risk patients:
Third, we are combining the best of both worlds of IO + TKI doublet therapy, namely deep and sustained responses:
Why we have not succeeded so far? Dr. Albiges then discussed the COSMIC-313 trial,1 which was a phase 3 trial of cabozantinib in combination with ipilimumab + nivolumab versus ipilimumab + nivolumab. Patients with advanced clear-cell renal-cell carcinoma who had not previously received treatment and had intermediate or poor prognostic risk were enrolled into COSMIC-313. Patients were randomly assigned to receive cabozantinib daily in addition to nivolumab and ipilimumab or matched placebo in addition to nivolumab and ipilimumab. Patients then received nivolumab maintenance therapy (480 mg once every 4 weeks) for up to 2 years. The trial design for COSMIC-313 is as follows:
The primary endpoint was progression-free survival, as determined by a blinded independent review according to RECIST, v1.1. The secondary endpoint was overall survival, assessed in all patients who had undergone randomization. This trial had 855 patients who underwent randomization. Among the first 550 patients that were randomized, the probability of progression-free survival at 12 months was 0.57 in the ipilimumab + nivolumab + cabozantinib group and 0.49 in the ipilimumab + group (HR 0.73, 95% CI 0.57 to 0.94):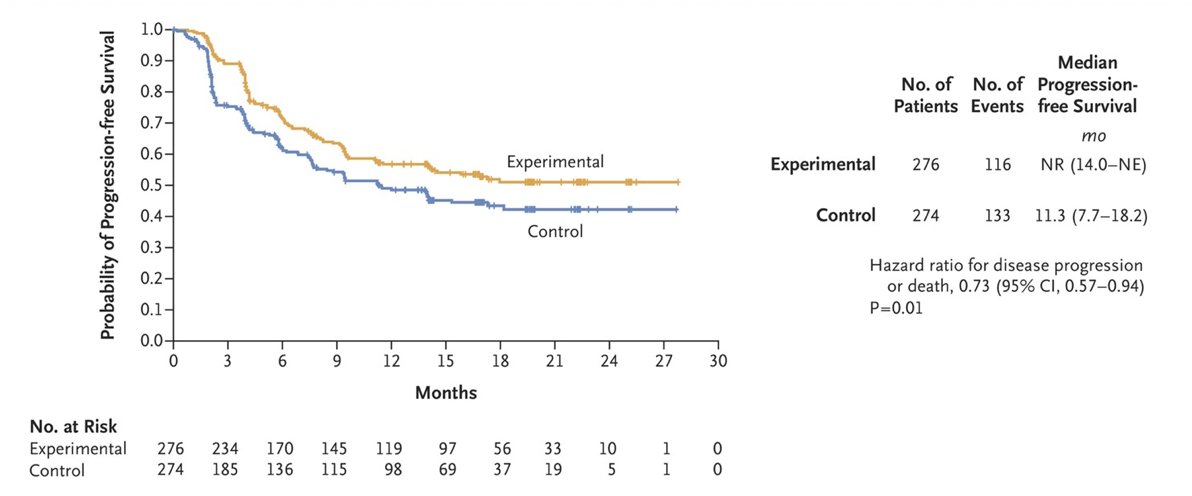
Additionally, more patients on triplet therapy (90%) compared to doublet therapy (75%) had tumor shrinkage:
There was an increase in the number of patients achieving a partial response, but no difference (3% each group) for complete response, and fewer patients with a deep response. Thus, no difference in cure rate. Grade 3 or 4 adverse events occurred in 79% of the patients in the experimental group and in 56% in the control group, likely secondary overlapping toxicity in the triplet arm:
As such, Dr. Albiges notes that although there was benefit in early endpoints, toxicity failed to ensure drug exposure: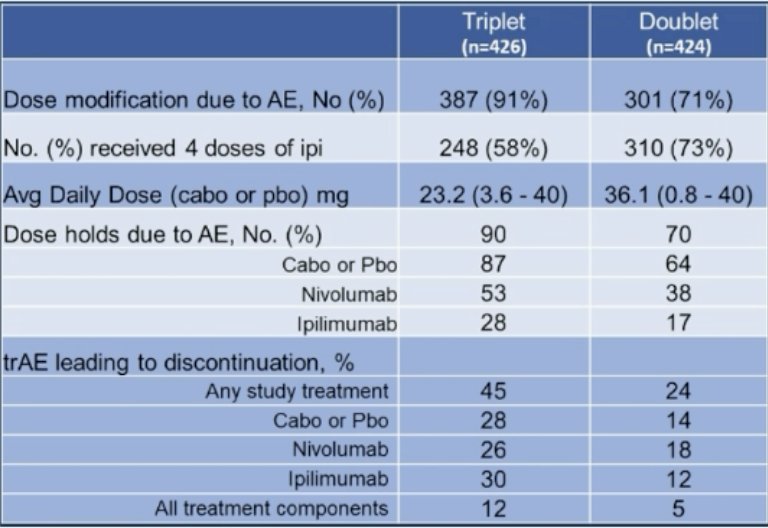
So, what is needed now? First, we need new mechanisms of action. MK6482-012 is randomizing untreated metastatic RCC patients to pembrolizumab + belzutifan + lenvatinib versus pembrolizumab + quavonlimab + lenvatinib versus pembrolizumab + lenvatinib. Moreover, many ongoing trials are looking at new TKIs, new IOs, and new mechanisms of action in the first-line disease space: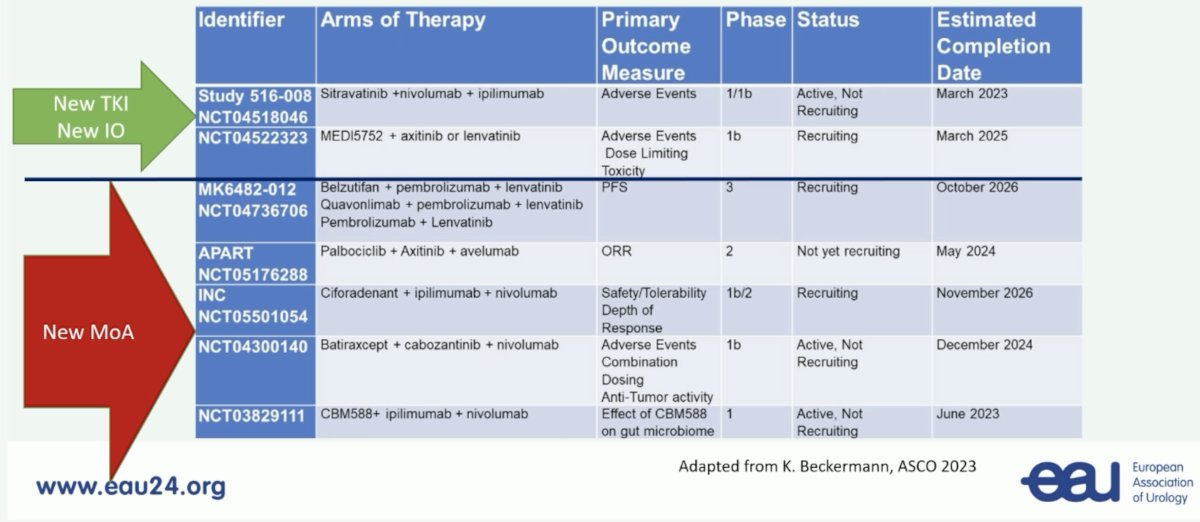
Secondly, we need an adaptive approach. This is being addressed in the PDIGREE clinical trial, which was activated in May 2019: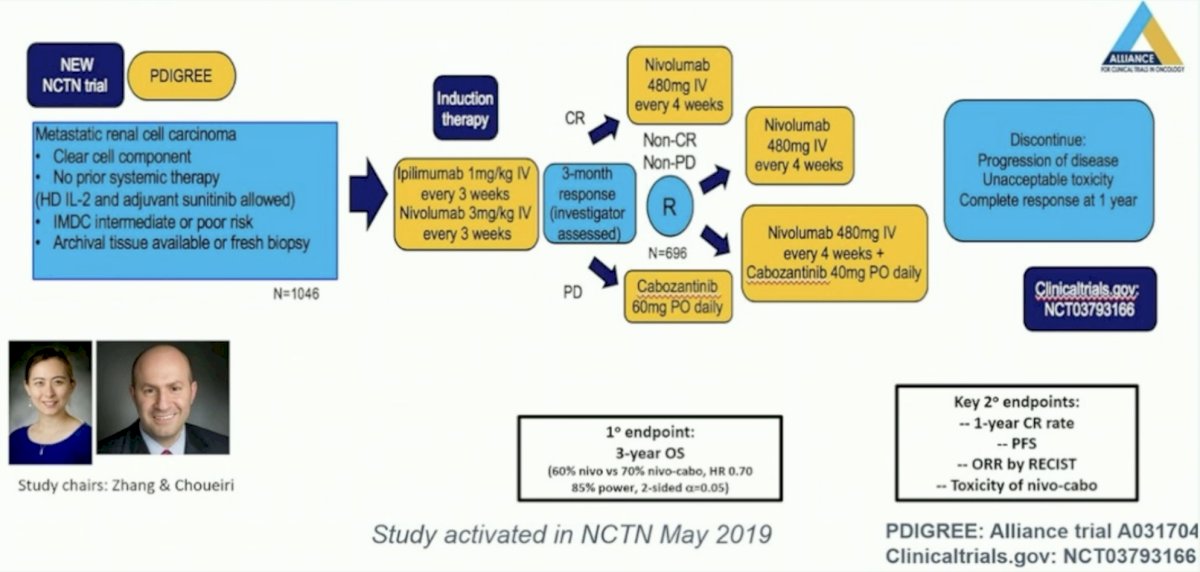
Third, we need improved patient selection. This is being addressed in several biomarker driven trials, including the BIONIKK and OPTIC RCC clinical trials:
Another pragmatic trial, in Europe, is a first line randomized study platform to optimize treatment in patients with metastatic RCC based on PDL1 expression: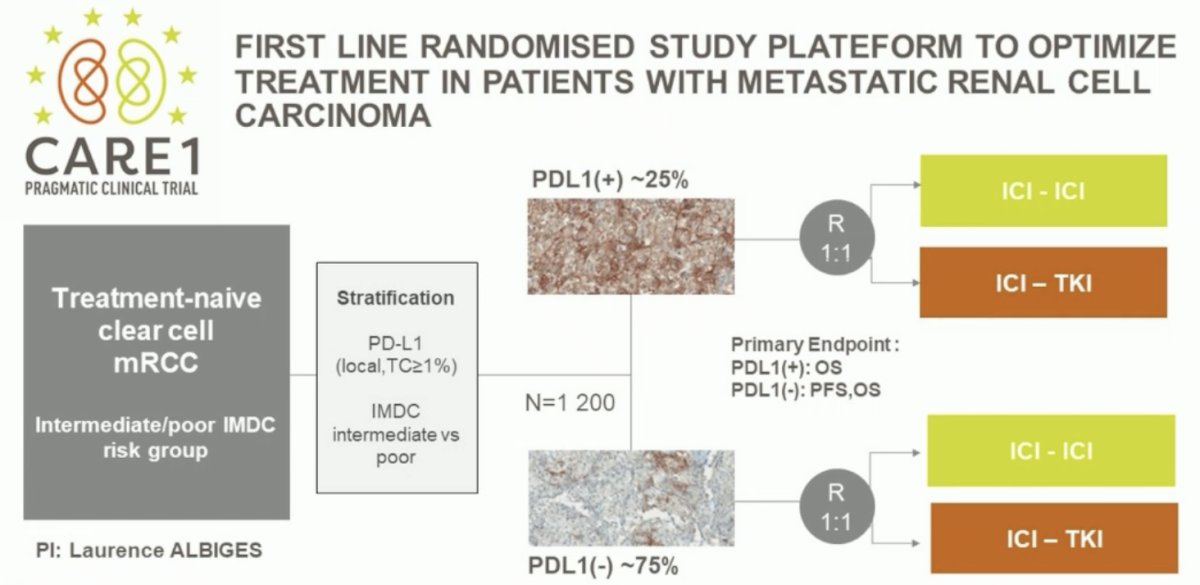
Dr. Albiges concluded her presentation discussing that for first line metastatic kidney cancer we should be using triplet combinations at any cost by emphasizing that in particular for young patients with aggressive disease, we are aiming for a cure.
Presented by: Laurence Albiges, MD, PhD, Gustave Roussy, Villejuif, France
Written by: Zachary Klaassen, MD, MSc - Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, WellStar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
References:
- Choueiri TK, Powles T, Albiges, et al. Cabozantinib plus Nivolumab and Ipilimumab in Renal Cell Carcinoma. N Engl J Med. 2023 May 11;388(19):1767-1778.


