(UroToday.com) The 2024 European Association of Urology (EAU) annual meeting featured a plenary session on personalized approaches in high-risk and metastatic prostate cancer, and a presentation by Dr. Fred Saad discussing that PARP inhibitors should not be reserved only for patients with alterations in DNA repair genes. Dr. Saad notes that when looking at the phase 3 trials in mCRPC across the last decade or so, the improvement in overall survival between experimental and control arms generally ranges from +2.9 to +4.8 months of improved survival:
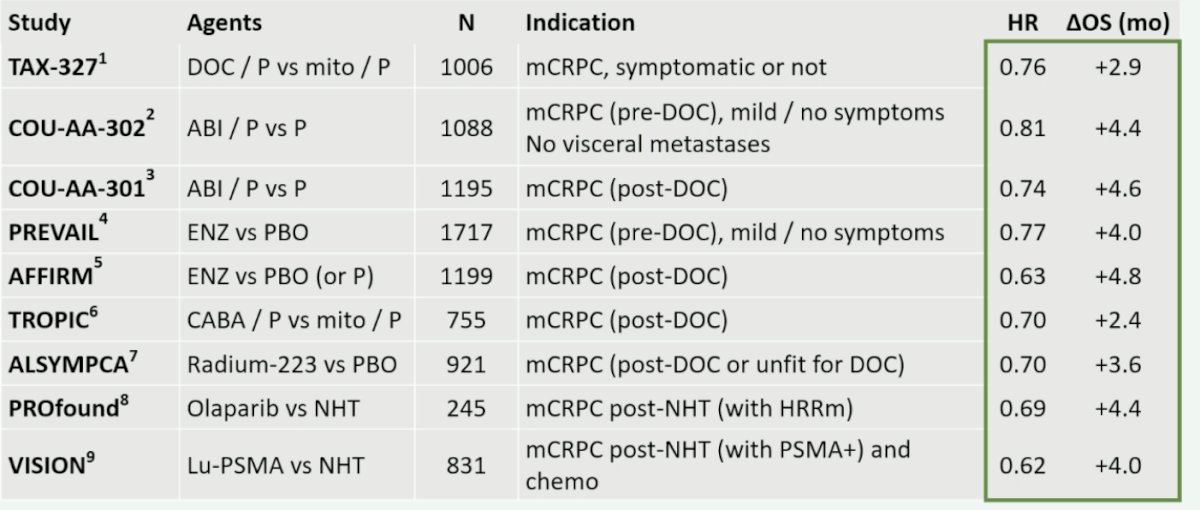
Notably, all of these studies had an inactive control arm. When combining a PARP inhibitor with an NHA, PARP inhibition is reported to increase the activity of the NHA via AR-dependent transcription, and the NHA is reported to induce HRR deficiency and increase susceptibility to PARP inhibition. This provides a combined effect leading to anti-tumor activity in HRR mutated and non-HRR mutated prostate cancer:

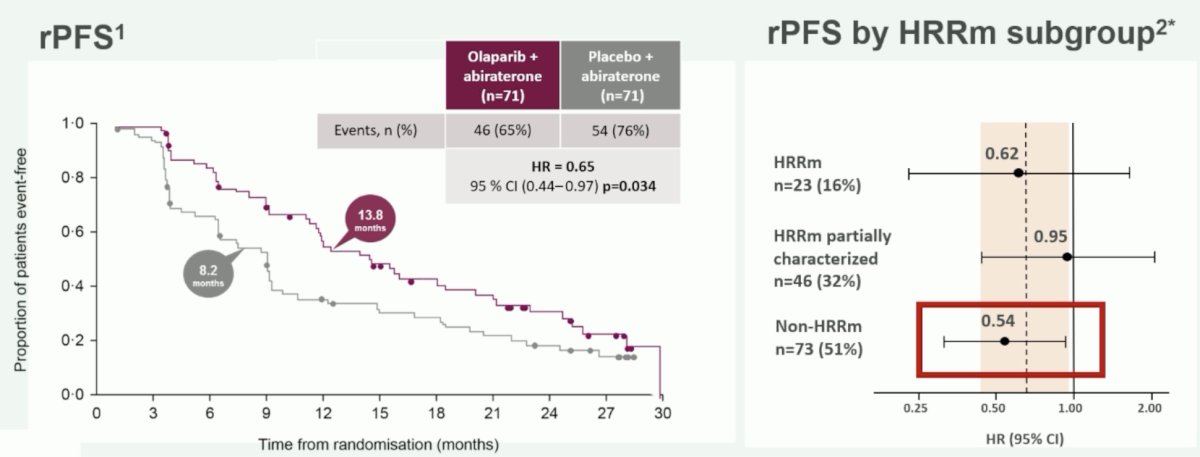
In the phase 2 Study 08, there was a benefit of olaparib + abiraterone versus placebo + abiraterone (post docetaxel), particularly when assessing the subgroup of non-HRR mutated patients (HR 0.54):
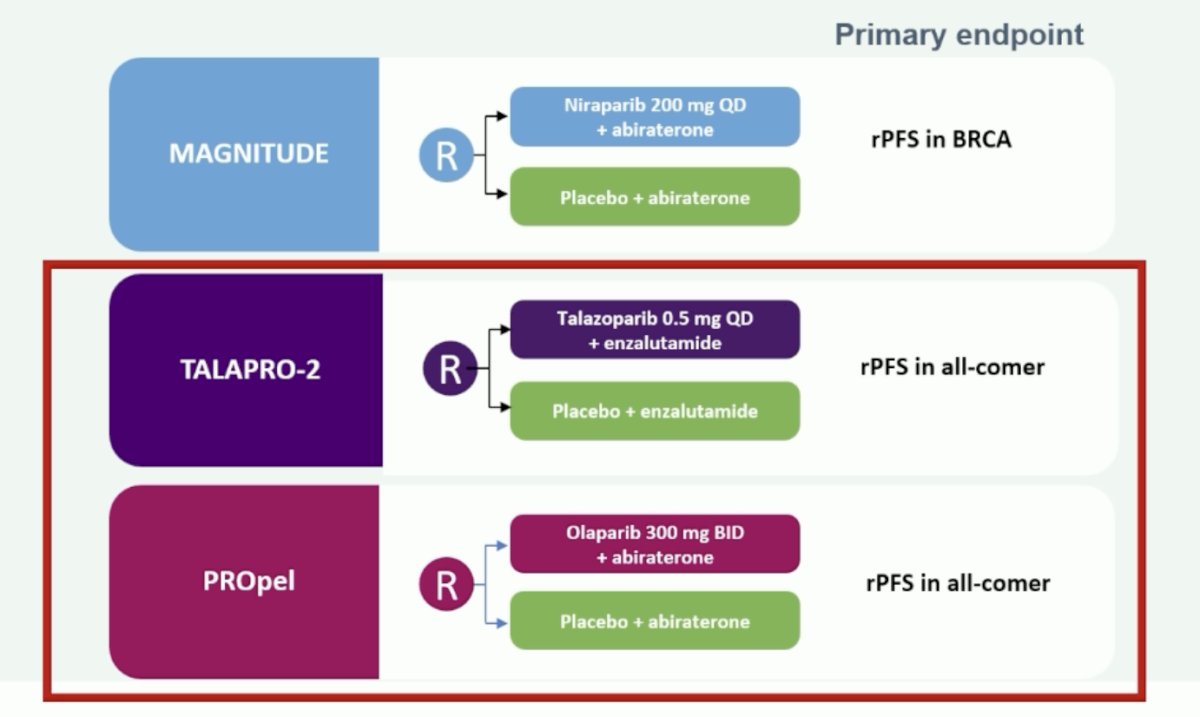
Dr. Saad summarized the three key trials, MAGNITUDE [1], TALAPRO-2 [2], and PROpel [3], by emphasizing an rPFS benefit in all comers seen in the TALAPRO-2 and PROpel trials:
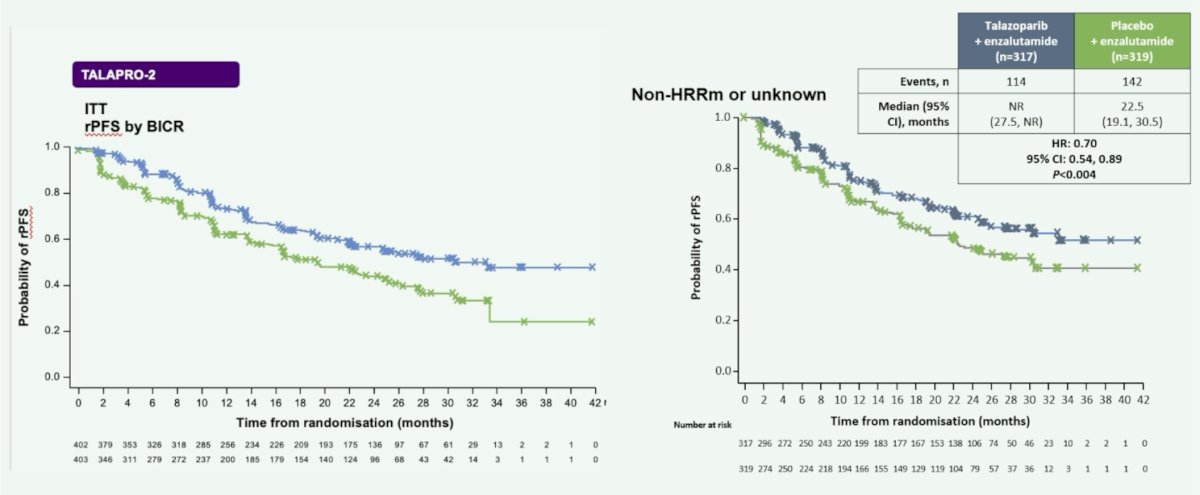
In TALAPRO-2, talazoparib + enzalutamide compared to enzalutamide alone improved rPFS in the intention to treat and non-HRR mutated (HR 0.70, 95% CI 0.54-0.89) populations:
Similarly, in PROpel, olaparib + abiraterone compared to abiraterone alone improved rPFS in the intention to treat (median OS improvement of 11.2 months) and non-HRR mutated subgroup (median OS improvement of 8.5 months):
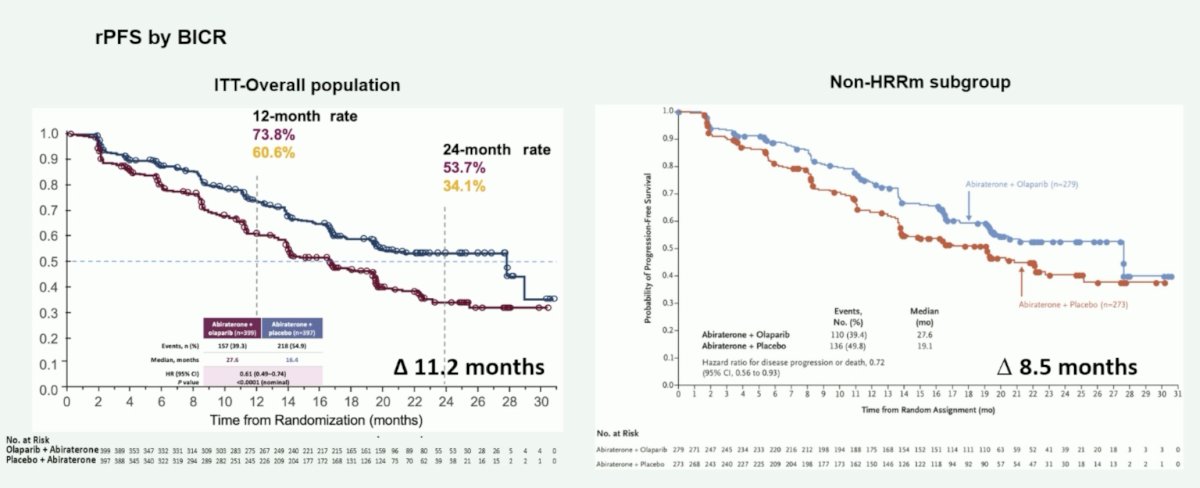
Moreover, in PROpel, abiraterone + olaparib improved PSA response rate (7.2 month improvement) and time to PSA progression (median 8.9 month improvement) in the non-HRR mutated population. To date, the overall survival benefit in the intention to treat population is 7.4 months and 2.2 months in the non-HRR mutation population:

Dr. Saad concluded his presentation by discussing that PARP inhibitors should not be reserved only for patients with alterations in DNA repair genes with the following conclusions:
- Three phase 3 trials confirm that PARP inhibitor + NHA is most effective in first-line mCRPC patients with BRCA mutation/HRR mutation
- Two phase 3 trials confirm that PARP inhibitor + NHA is effective in patients without HRR mutations/BRCA mutations
- Deciding who we treat that are non-HRR mutation/BRCA mutation or unknown should depend on risk factors for early progression on NHT alone and shared decision making with the patient
Presented by: Fred Saad, MD, FRCS, Centre de l’Université de Montreal, Université de Montreal, Montreal, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 European Association of Urology (EAU) annual congress, Paris, France, April 5th - April 8th, 2024
Related Content:
EAU 2024: PARP-Inhibitors Only for Patients with Alterations in DNA Repair Genes: Yes
EAU 2024: PARP-Inhibitors Only for Patients with Alterations in DNA Repair Genes: The Guidelines' View


