(UroToday.com) The 37th Annual European Association of Urology Congress held in Amsterdam, the Netherlands between July 1st, and 4th 2022 was host to prostate cancer “Game-Changing Session”. Professor Jan Philipp Radtke led a discussion session following Dr. Tombal’s presentation: “Overall Survival with Darolutamide versus placebo in combination with androgen deprivation therapy and docetaxel by stratification factors in the phase 3 ARASENS trial”.
The ARASENS trial demonstrated that addition of darolutamide to ADT and docetaxel significantly improved overall survival in patients with metastatic hormone-sensitive prostate cancer (mHSPC) by 32% (HR: 0.68, 95% CI: 0.57 – 0.80, p<0.001).
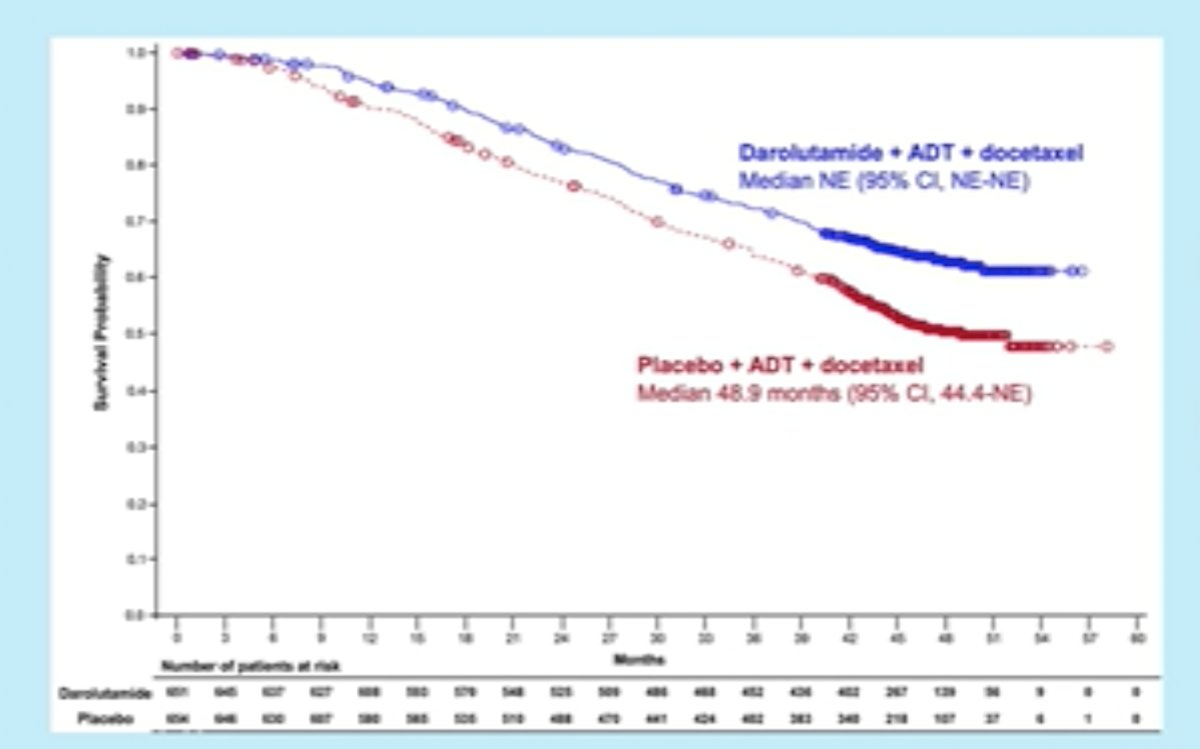
Addition of darolutamide similarly prolonged time to development of castration resistance (HR: 0.36, 95% CI: 0.30 – 0.42, p<0.001).1
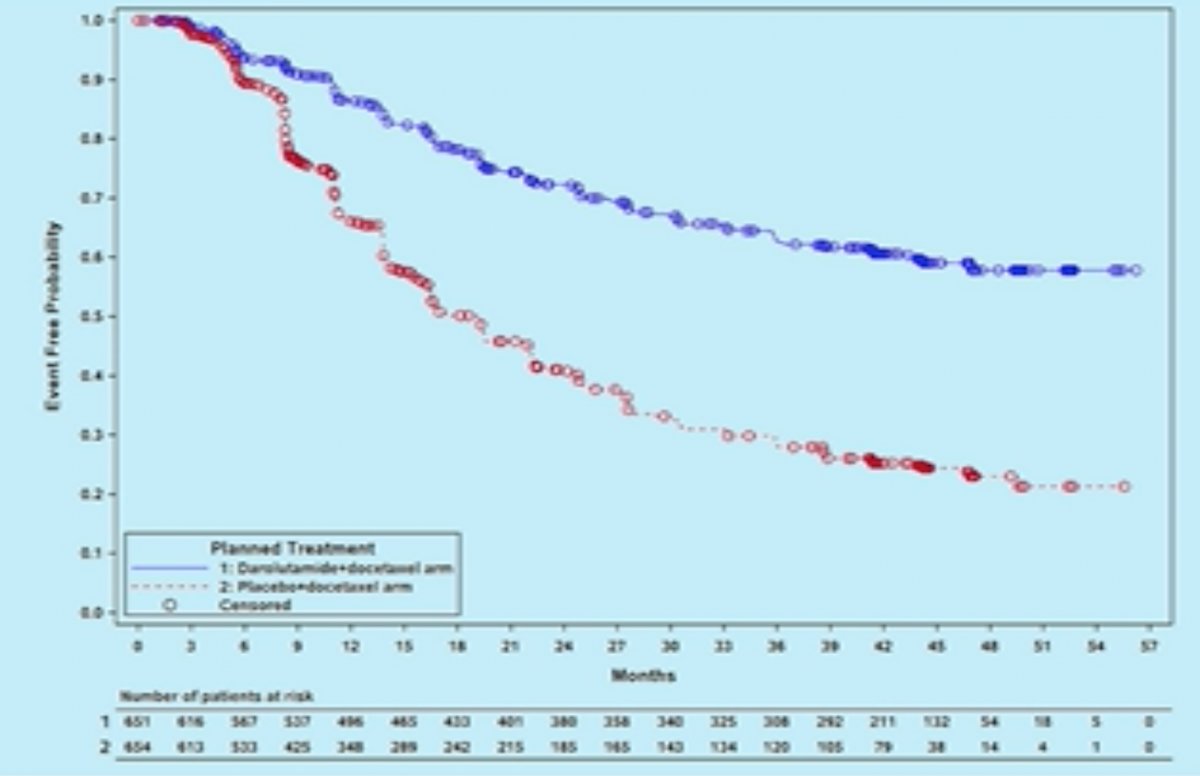
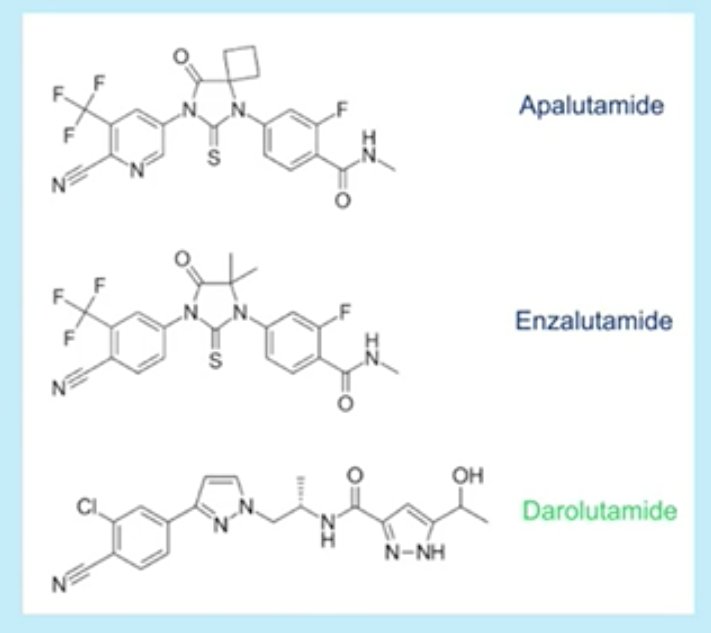
What was the rationale for addition of darolutamide to a regimen consisting of ADT and docetaxel? Preclinical models have demonstrated that targeting the androgen receptor can enhance taxane-induced cellular apoptosis. Amongst the androgen receptor inhibitors (ARI), darolutamide is a potent agent with a favorable safety profile, with most adverse events commonly associated with ARIs showing a <2% difference between darolutamide and placebo in patients with non-metastatic CRPC.

Structurally, darolutamide incorporates two pharmacologically active diastereomers and thus penetration through the blood-brain barrier is negligible. Darolutamide has no clinically relevant inhibition of most common cytochrome pathways (CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, or 3A4). For the most part, darolutamide has also shown to be resistant to androgen receptor mutations.
Professor Radtke next addressed whether the addition of darolutamide to ADT and docetaxel showed consistent benefits across the following subgroups:
- Synchronous versus metachronous mHSPC
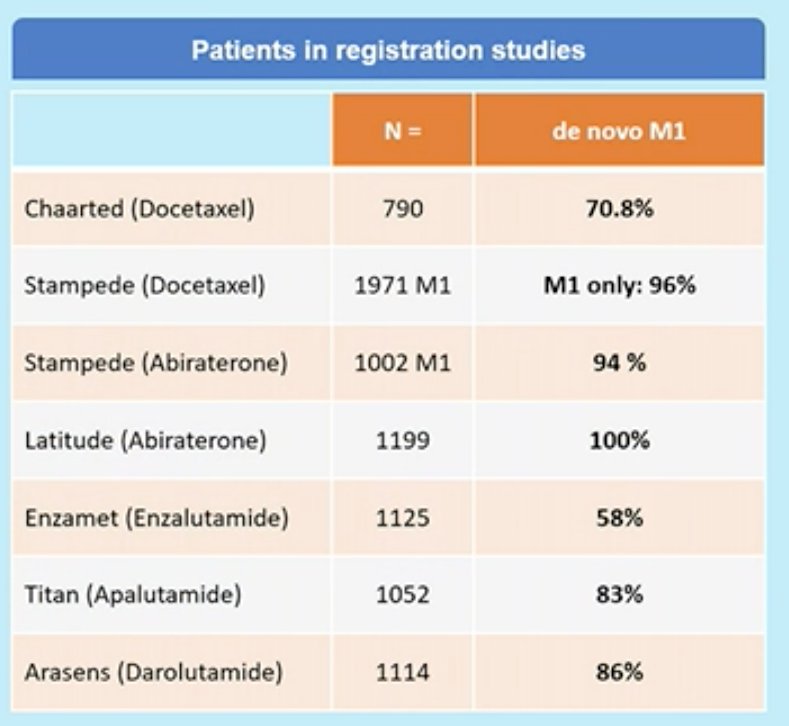
- 86% of patients in the ARASENS trial had the synchronous disease (i.e. de novo metastasis)
- High versus low metastatic burden
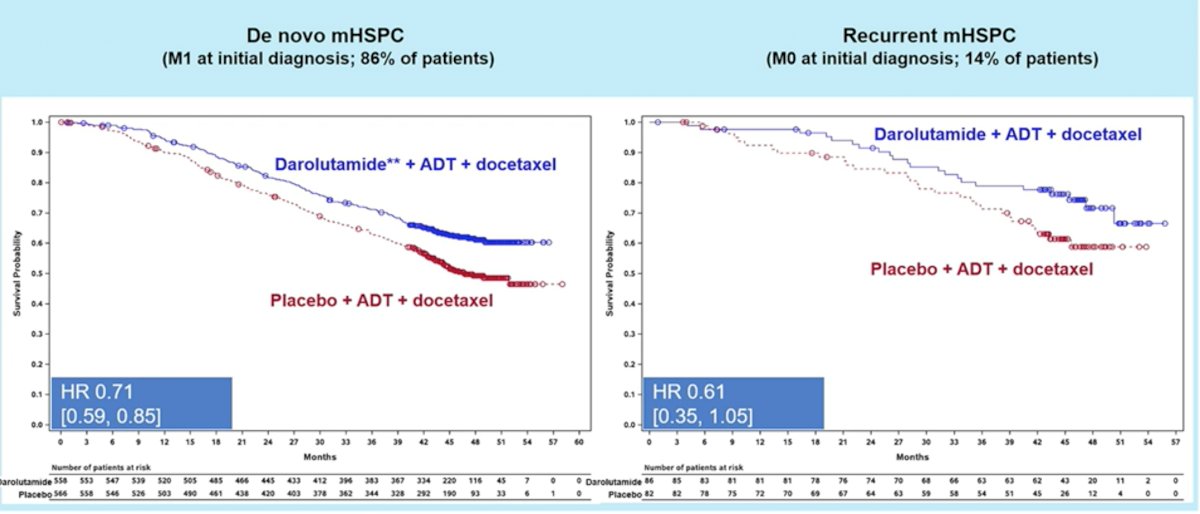
Addition of darolutamide consistently showed OS benefits in both synchronous (HR: 0.71, 95% CI: 0.59 – 0.85) and metachronous mHSPC (HR: 0.61, 95% CI: 0.35-1.05). Of note, OS benefit did not reach statistical significance in the metachronous subgroup likely secondary to the smaller subgroup sample size (14% of patients in the ARASENS trial).
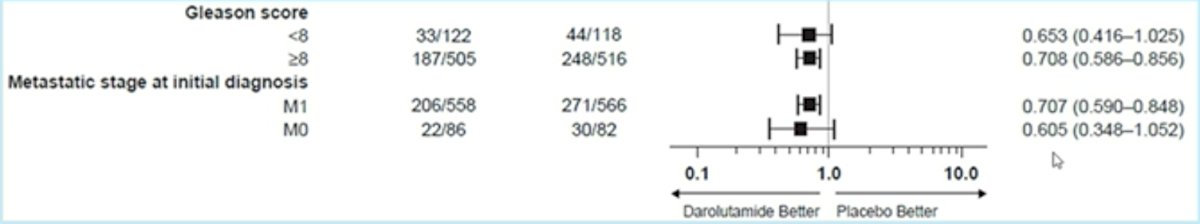
With regards to tumor burden, darolutamide showed OS benefits in patients with bone metastases (M1b, HR: 0.67, 95% CI: 0.55 – 0.81) and visceral metastases (M1c, HR: 0.79, 95% CI: 0.55 – 1.14). Notably, darolutamide appeared to be of limited benefit in patients with non-regional LN involvement (i.e. M1a, HR: 0.65, 95% CI: 0.19 – 2.25). As demonstrated by Dr. Tombal in the prior lecture, darolutamide addition improved OS across ALP level subgroups (above or below the upper limit of normal).
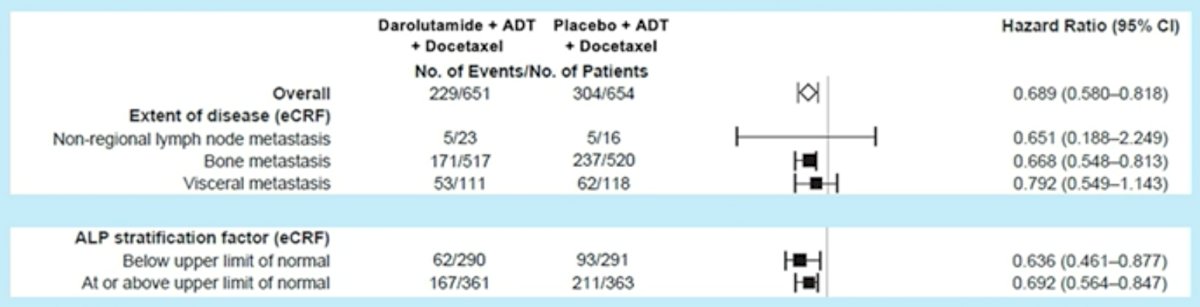
Professor Radtke notes that risk (LATITUDE) and volume (CHAARTED) subgroup outcomes have not been reported yet, and given the current data, high-volume, high-risk subgroups can only be partially “constructed”.
The adverse effect profile was favorable for addition of darolutamide with no significant difference in the rates of serious adverse events between the two groups.
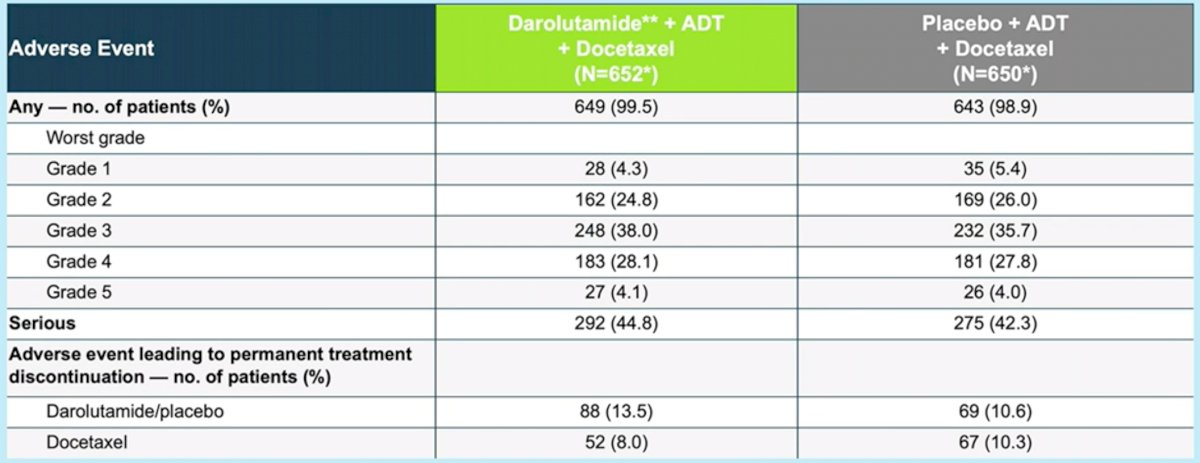
Professor Radtke ended his presentation with the following points:
- Subgroup analysis according to risk (LATITUDE) or volume (CHAARTED) have not yet been presented
- Tumor burden risk stratification by ALP levels and bone metastases demonstrated consistent benefits in favor of darolutamide addition
- We await the results of ARANOTE (Darolutamide + ADT versus ADT in mHSPC) in 2024
- If you want to give your patient chemotherapy, ARASENS suggests that patients benefit from the addition of darolutamide
- Need to consider the sequence of therapy and side effect profile when considering doublet versus triplet therapy
Presented by: Professor Jan Philipp Radtke, MD, MBA, Department of Urology, Heinrich-Heine University Düsseldorf, Germany
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
References:


