(UroToday.com) The 37th Annual European Association of Urology Congress held in Amsterdam, Netherlands between July 1st, and 4th 2022 was host to a prostate cancer “Game Changing Session”. Dr. Bertrand Tombal discussed results from the Phase 3 ARASENS trial evaluating overall survival with darolutamide versus placebo in combination with androgen deprivation therapy (ADT) and docetaxel by stratification factors.
The ARASENS trial is a randomized, double-blind, placebo-controlled global phase 3 trial of patients with metastatic hormone-sensitive prostate cancer (mHSPC) with ECOG performance status of 0 or 1 who were randomized in a 1:1 fashion to either Darolutamide 600 mg or placebo twice daily + ADT + docetaxel (75 mg/m2 x 6 weeks). This trial included 1,305 patients and data cut-off was October 25, 2021. The primary endpoint was OS, with the primary analysis planned to occur after 509 deaths.
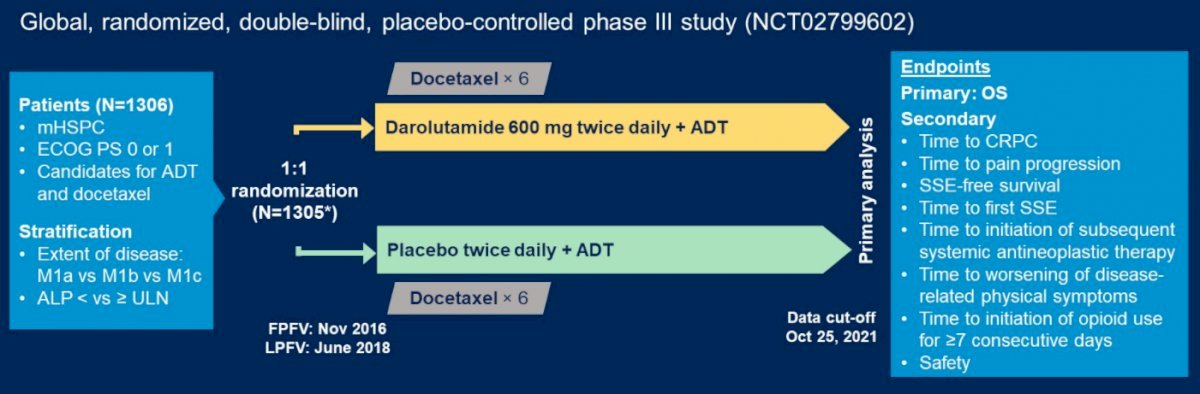
What was the rationale for Darolutamide use versus other androgen receptor inhibitors? Darolutamide is a structurally distinct and highly potent ARI that does not cross the blood-brain barrier. In the ARASENS trial, addition of darolutamide to ADT and docetaxel was shown to significantly reduce overall mortality by 32.5% (HR: 0.68, 95% CI: 0.57 – 0.80, p<0.001).
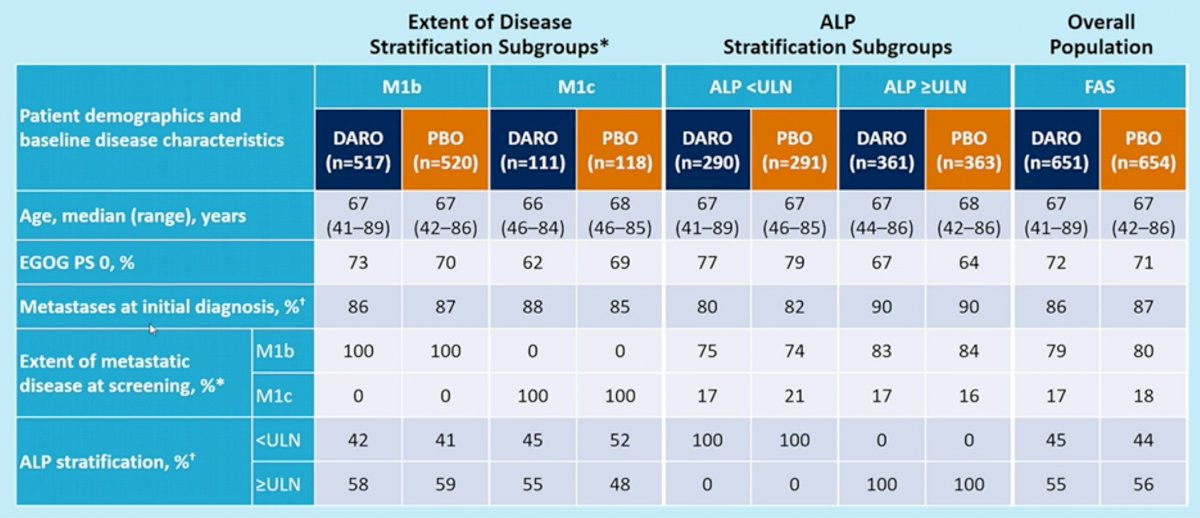
The GETUG-AFU-15 trial has demonstrated that alkaline phosphatase levels (ALP), a surrogate for bony metastasis volume, is a poor prognostic marker for OS in patients with mHSPC. Dr. Tombal’s group thus sought to investigate whether Darolutamide addition to ADT and docetaxel would improve OS in prespecified ARASENS stratification subgroups based on the extent of disease and ALP levels.
Baseline characteristics were well-balanced between treatment arms in all stratification subgroups (overall cohort, M1b, M1c, ALP < upper limit of normal, ALP >= upper limit of normal). Notably, the majority of patients had evidence of synchronous mHSPC.
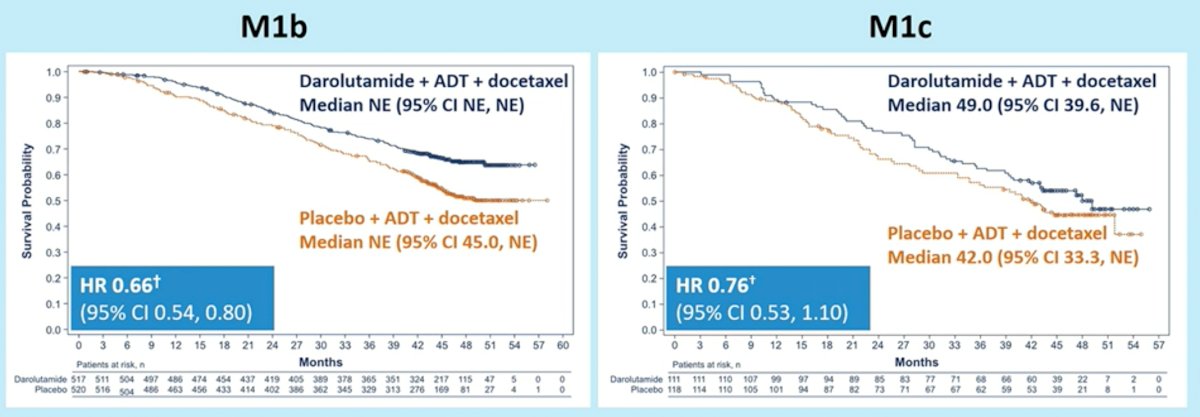
Disease stratification by metastatic burden (M1b versus M1c) demonstrated consistent benefits for darolutamide addition to ADT and docetaxel. In the M1b subgroup, HR for OS was 0.66 (95% CI: 0.54 – 0.80). In the M1c group, HR for OS was 0.76 (95% CI: 0.53 – 1.10), with median OS of 49.0 months in the darolutamide arm and 42.0 months in the placebo arm.
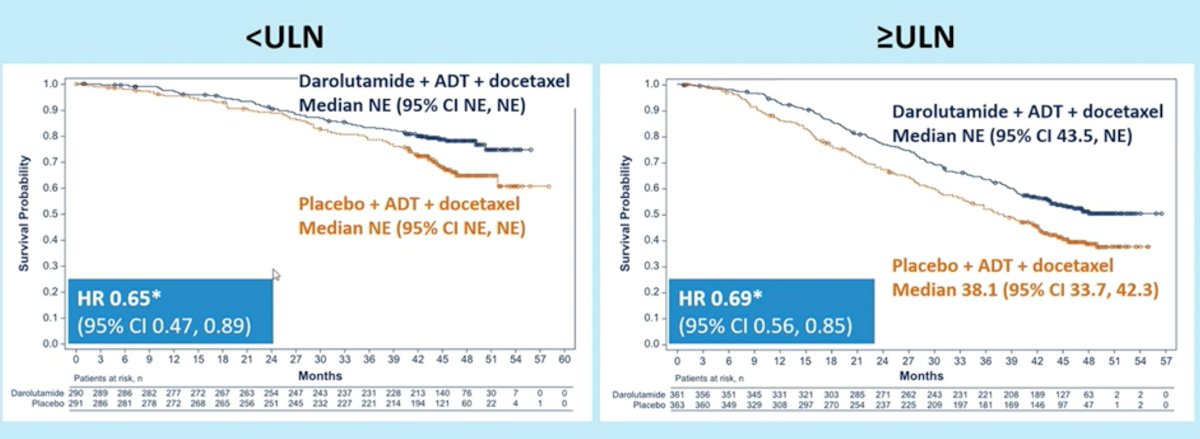
Disease stratification by ALP levels (<ULN and >=ULN) demonstrated similar findings. In patients with ALP levels <ULN, addition of darolutamide to ADT and docetaxel was associated with improved OS with a HR of 0.65 (95% CI: 0.47 – 0.89). In the ALP >= ULN subgroup, addition of Darolutamide was similarly associated with improved OS with a HR of 0.69 (95% CI: 0.56 – 0.85).
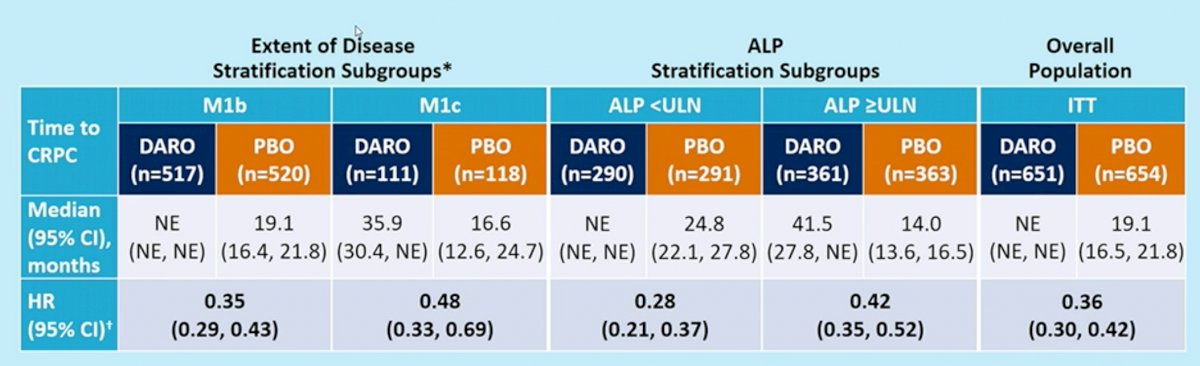
Consistent findings were seen with the surrogate endpoint of time to castration resistance, where addition of darolutamide significantly reduced the hazard of developing castration resistance (HRs: 0.28 – 0.48).
The safety profile of Darolutamide was favorable with the incidence and severity of treatment-emergent adverse events similar in both treatment arms and across all subgroups. The rate of serious adverse events ranged between 39% and 53%. Serious side effects leading to permanent discontinuation of Darolutamide/placebo ranged between 9% and 15%.
Presented by: Dr. Bertrand Tombal, MD, PhD, Chairman of the Department of Surgery and Full Professor of Urology at the Université catholique de Louvain, Ottignies-Louvain-la-Neuve, Belgium
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2022 European Association of Urology (EAU) Annual Hybrid Meeting, Amsterdam, NL, Fri, July 1 – Mon, July 4, 2022.
Related Content: EAU 2022: Phase 3 ARASENS Trial Discussion - Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer


