In defining risk strata of non-muscle invasive bladder cancer, Dr. Grimm highlighted the European Organization for Research and Treatment of Cancer (EORTC) risk calculator which utilizes the number of tumors, size, recurrences, stage (T category), concomitant carcinoma in situ, and grade. These are associated with the probability of recurrence and progression. More up to date version of the European Association of Urology (EAU) guidelines have defined high-risk tumors as any of the following: T1 disease, high-grade disease, carcinoma in situ, and multiple, large, recurrent Ta low-grade tumors.
Dr. Grimm then highlighted the recommended BCG treatment approach, based on the Lamm data. There have been multiple efforts to reduce the symptom burden associated with BCG, including dose reductions and decrease dose frequency. The recently published NIMBUS trial compared a reduced frequency of BCG instillation with standard frequency.
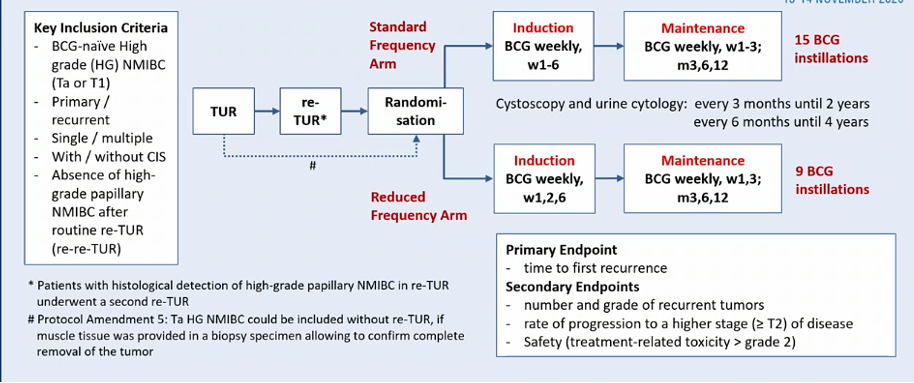
This study was prematurely stopped on the basis of evidence of worse recurrence-free survival among patients in the reduced frequency arm.

A post-hoc analysis of this trial with stratification according to EORTC and Club Urológico Español de Tratamiento Oncológico (CUETO) risk category demonstrated that the EORTC risk calculator overestimated the probability of recurrence compared to the standard therapy arm of the NIMBUS trial, and the CUETO risk calculator also over-estimated the probability of recurrence, at least among high-risk individuals.
Dr. Grimm highlighted that, among patients with BCG unresponsive disease, current guidelines recommend radical cystectomy with bladder preservation strategies, preferably within the context of clinical trials, and could be offered to those who refuse cystectomy. In this context, he highlighted data from KEYNOTE-057 which provided the evidence necessary for the registration of pembrolizumab as a treatment option in this disease space. This single-arm trial demonstrated a complete response rate of 40.6% with persistent disease in 42%, recurrent disease in 6%, and stage progression in 9% with a median duration of response of 16 months among those with a complete response. No patients progressed to muscle-invasive disease.
He then highlighted a number of ongoing studies examining immunotherapy approaches with BCG in patients with high-risk non-muscle invasive disease including KEYNOTE-676, POTOMAC, CheckMate-9UT, and CheckMate7G8.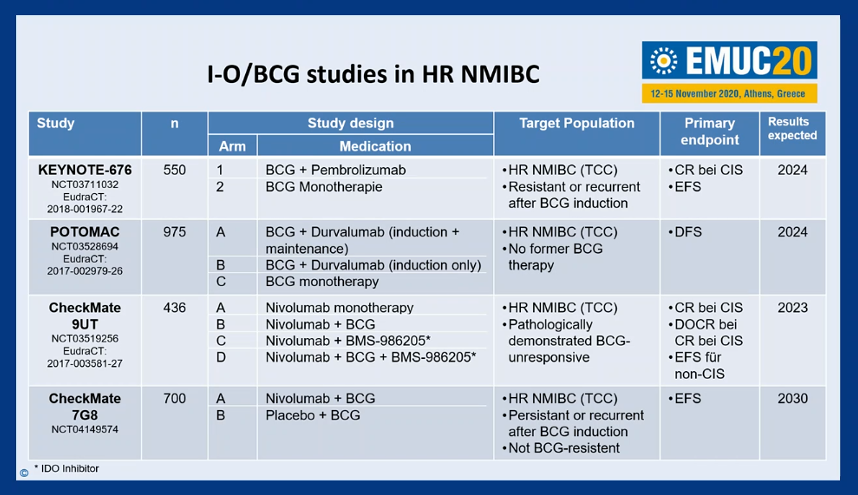
He then changed direction to discuss nadofaragene firadenovec, an intravesical therapy relying on a recombinant adenovirus to deliver gene therapy. A phase II trial demonstrated 35% recurrence-free survival at 12-months. Based on data presented at ASCO 2020, phase III data of this agent demonstrated a 53% complete response rate among patients with CIS, with somewhat higher rates among patients with papillary disease (73%). These responses appeared to be durable with approximately half of the patients who achieved complete response at 3 months maintaining this at 12 months. Progression to muscle-invasive disease occurred in 5% of patients, though many (~66%) had high-grade non-invasive recurrences.
Summarizing, he highlighted that available risk calculators over-estimate the rates of recurrence and progression; BCG remains the standard first-line approach and is associated with quite low rates of recurrence, and there are many recently completed and ongoing trials of novel approaches in patients with BCG unresponsive disease.
Presented by: Marc-Oliver Grimm, MD, Professor, Otto-Schott-Institut der Friedrich-Schiller-Universität Jena, Jena, Germany
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, @WallisCJD on Twitter at the 12th European Multidisciplinary Congress on Urological Cancers (EMUC) (#EMUC20 ), November 13th - 14th, 2020
Related Content: ASCO GU 2020: Treatment of High-Grade Non-Muscle Invasive Bladder Carcinoma: Results of the Phase III Clinical Trial (NIMBUS)
ASCO 2020: Pembrolizumab for the Treatment of Patients with Bacillus Calmette-Guérin Unresponsive, High-Risk Non–Muscle-Invasive Bladder Cancer: Over Two Years Follow-Up of KEYNOTE-057
ASCO GU 2020: Safety and Efficacy of Intravesical Nadofaragene Firadenovec for patients with High-Grade, BCG Unresponsive Nonmuscle Invasive Bladder Cancer - Phase 3 Trial Results


