Munich, Germany (UroToday.com) Enzalutamide is an androgen signaling inhibitor which prevents androgen receptor nuclear translocation and DNA binding, therefore leading to cellular apoptosis. Enzalutamide has been largely studied in the metastatic castration-resistant population, demonstrating an overall survival benefit for patients before and after chemotherapy1,2. In an open-label, single arm, phase II study, enzalutamide was also found to be well tolerated in patients with castration-sensitive prostate cancer, with 92.5% of patients achieving at 80% PSA decline or greater by week 25 of therapy3. However, it is unknown if enzalutamide is more effective than bicalutamide in combination with standard ADT for patients with metastatic castration sensitive prostate cancer.
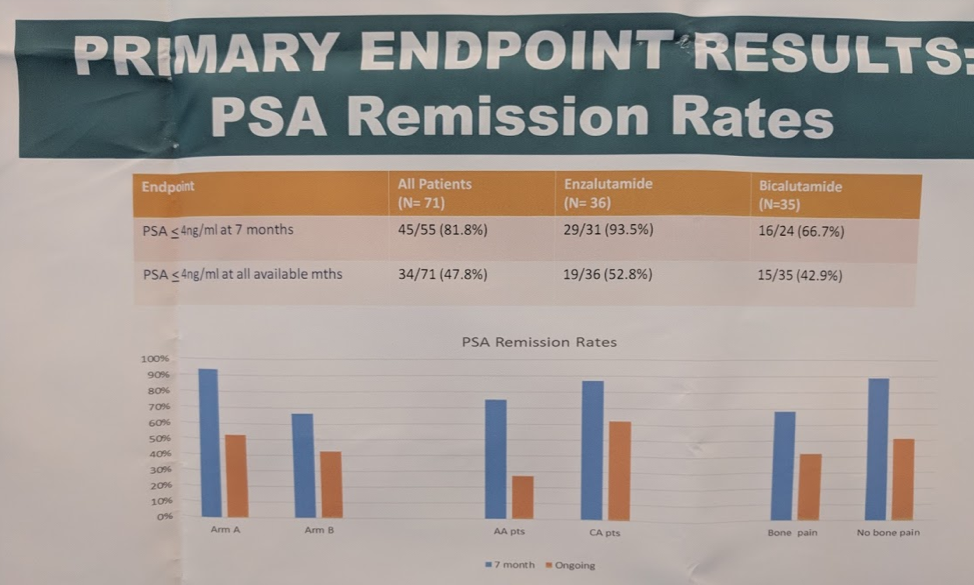
This study randomized 71 men to receive ADT in combination with either enzalutamide or bicalutamide and the primary endpoint was a seven month PSA remission, with a PSA nadir less than 4 ng/mL.

The patient population was 58% Caucasian and 41% African American. Patient characteristics appeared balanced amongst the enzalutamide and bicalutamide cohorts by age, race, performance status, bone mets, and measurable disease.

94% of patients reached the primary endpoint of PSA remission with enzalutamide, compared with 67% of patients on bicalutamide. There were more deaths in the bicalutamide arm compared with the enzalutamide arm (4 (11%) vs 13 (37.1%)). Amongst African American patients in particular, 100% of patients reached the primary endpoint of 7 month PSA remission, compared with 46 % of patients on bicalutamide.
In terms of correlative testing, changes in tumor tissue expression of ERG, CXCR4, and AKR1C3 were reported. There did not appear to be a difference in ERG expression or AKR1C3 pre and post-treatment. CXCR4 increased post-treatment.

This study demonstrated that enzalutamide is able to increase the number of patients with PSA nadir of <4 at 7 months in patients with metastatic castration sensitive prostate cancer. It is remarkable that 100% of African American patients achieved the primary endpoint, suggesting that African Americans may have a greater PSA response to Enzalutamide than Caucasian patients. Similar findings for Abiraterone have been suggested by Abi Race, a prospective, multicenter study of African American and Caucasian patients with mCRPC treated with abiraterone acetate and prednisone4. Longer follow-up will be necessary to determine if a benefit in overall survival is seen with enzalutamide compared with bicalutamide in this setting.
Presented By: Ulka N. Vaishampayan, MD, Karmanos Cancer Institute, Detroit, US
Written By: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu
References:
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. The New England journal of medicine 2014;371:424-33.
- Scher HI, Fizazi K, Saad F, et al. Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy. New England Journal of Medicine 2012;367:1187-97.
- Tombal B, Borre M, Rathenborg P, et al. Enzalutamide monotherapy in hormone-naive prostate cancer: primary analysis of an open-label, single-arm, phase 2 study. The lancet oncology 2014;15:592-600.
- George DJ, Heath EI, Sartor AO, et al. Abi Race: A prospective, multicenter study of black (B) and white (W) patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC) treated with abiraterone acetate and prednisone (AAP). American Society of Clinical Oncology; 2018.


