(UroToday.com) Urothelial carcinoma has a substantial impact on patient qualify of life. Patients may experience a multitude of disease-related symptoms, including pain, urinary frequency, physical changes, and mental health issues, with all affect quality of life. Additionally, treatment side effects of chemotherapy have been shown to reduce the quality of life in patients with advanced urothelial carcinoma. In the phase III JAVELIN Bladder 100 trial, avelumab (anti–PD-L1) first-line maintenance plus best supportive care significantly prolonged overall survival (primary endpoint) versus best supportive care alone in patients with advanced urothelial carcinoma without disease progression with first-line induction chemotherapy (HR 0.69, 95% CI 0.56-0.86).1 At the European Society of Medical Oncology (ESMO) 2020 Virtual Congress, Dr. Thomas Powles and colleagues reported patient-reported outcomes from the JAVELIN Bladder 100 study.
The trial schema for JAVELIN Bladder 100 is as follows:
In JAVELIN Bladder 100, patient-reported outcomes were a secondary endpoint and were assessed at baseline, on day 1 of each 4-week cycle, at the end of treatment/withdrawal visit, and at short-term follow-up visits (up to 90 days post-treatment). Two patient-reported outcome instruments were employed: National Comprehensive Cancer Network – Functional Assessment of Cancer Therapy Bladder Cancer Symptom Index-18 (FBlSI-18) and EuroQol 5 Dimensions 5 Levels (EQ-5D-5L). Descriptive, mixed-model, and time-to-deterioration (≥3-point decrease from baseline in the FBlSI disease-related symptoms–physical subscale for 2 consecutive assessments using the Kaplan-Meier method) analyses were conducted in both primary populations: all randomized patients and patients with PD-L1+ tumors.
Among all randomized patients in the avelumab plus best supportive care (n=350) and best supportive care alone (n=350) arms, completion rates for both patient-reported outcome instruments were >90% for the majority of the treatment period. Results from the descriptive analysis and mixed models over the treatment period in FBlSI-18 and EQ-5D-5L were similar between arms:
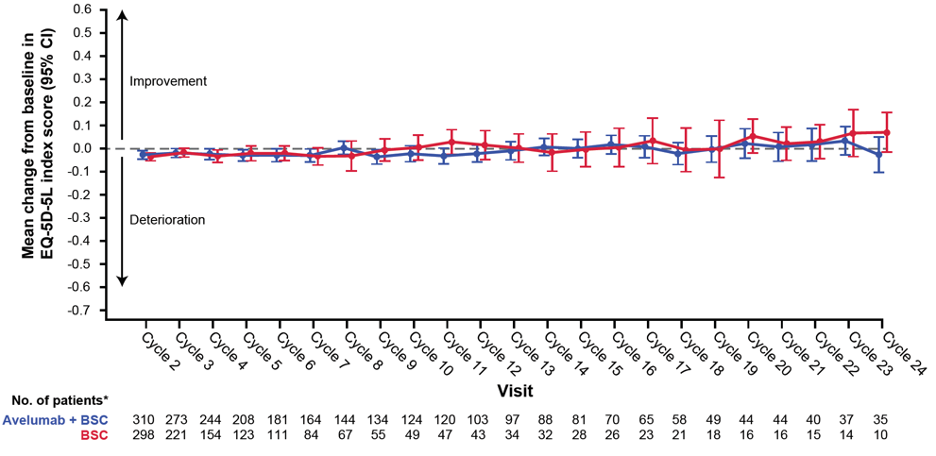
The hazard ratio for time to deterioration was 1.26 (95% CI 0.90-1.77; 1-sided p=0.91); median time to deterioration was not reached (95% CI 13.9 months to not estimable) with avelumab plus best supportive care and was 13.8 months (95% CI 12.9 months to not estimable) with best supportive care alone. However, the time to deterioration results should be interpreted with caution given that death and progression were not included in the event definition, and starting from cycle 2 substantially fewer patients were eligible to complete patient report outcome tools with best supportive care alone (n=155) than with avelumab plus best supportive care (n=206).
Additionally, patient-reported outcome results were consistent in the PD-L1+ population.
Dr. Powles concluded this presentation of patient-reported outcomes among patients in JAVELIN Bladder 100 with the following take home messages:
- Results from descriptive analyses and mixed models of validated patient-reported outcome instruments were similar for avelumab plus best supportive care versus best supportive care alone, both in the overall and PD-L1 positive populations
- Findings from JAVELIN Bladder 100 show that avelumab first line maintenance prolongs overall survival with no detrimental effect on clinically relevant patient-reported outcomes, supporting the use of avelumab as a new standard of care for patients with advanced urothelial carcinoma that has not progressed on first-line chemotherapy
Presented by: Thomas B. Powles, MBBS, MRCP, MD, Department of Medical Oncology, Barts Cancer Institute, Experimental Cancer Medicine Centre, Queen Mary University of London, St Bartholomew’s Hospital, London, United Kingdom
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020
Related Content:JAVELIN Bladder 100 Phase III Results: Maintenance Avelumab + Best Supportive Case vs BSC Alone After Platinum-Based First-Line Chemotherapy in Advanced Urothelial Carcinoma
LBA1 Discussion: ASCO 2020: Checkpoint Inhibition in Metastatic Urothelial Carcinoma: Timing is Everything
JAVELIN Bladder 100: Results of First-line Maintenance Therapy Plus Best Supportive Care Demonstrates Significant Prolonged OS in Advanced Urothelial Cancer - Cora Sternberg
JAVELIN Bladder 100: Avelumab for Previously Untreated Locally Advanced or Metastatic Urothelial Carcinoma - Thomas Powles


