In KEYNOTE-199, patients with or without prior abiraterone were eligible if they developed resistance to enzalutamide following prior response. Patients continued on enzalutamide and received pembrolizumab 200 mg IV Q3W for up to 2 years or until progression, toxicity, or withdrawal. The trial schema for KEYNOTE-199 is as follows:
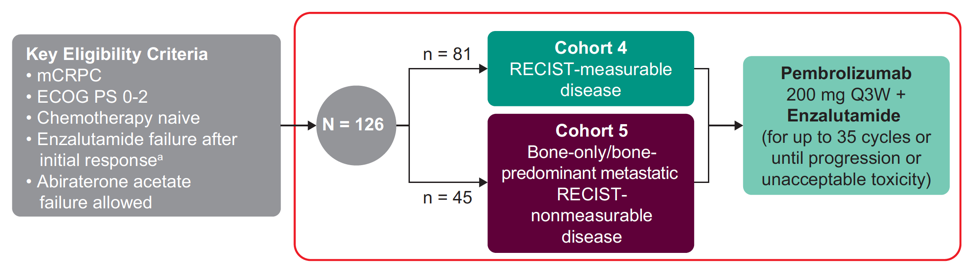
The primary endpoints were objective response rate (ORR) per RECIST v1.1 (for cohort 4) by blinded independent central review, duration of response (DOR) (for cohort 4), durable complete response (DCR), rPFS per PCWG3-modified RECIST, overall survival, time to cytotoxic chemotherapy, time to new anticancer therapy, and safety. The data cutoff date was June 24, 2019.
In this trial, 126 patients were treated including 81 in cohort 4 and 45 in cohort 5. The median time from enrollment to data cutoff was 11.8 months (range: 1.2 – 20.7) in cohort 4, and 18.6 months (range: 3.1-20.6) in cohort 5. The median PSA was 31 ng/mL (range: 0.4-1667) in cohort 4 and 19 ng/mL (range: 1-1750) in cohort 5. In cohort 4, the ORR was 12% (95% CI 6-22; 2 complete responses, 8 partial responses) and median DOR was 6.3 months (range: 2.5+ to 13.4); 4 responders had a response ≥6 months. The confirmed PSA response rate among patients who had a PSA measurement at baseline and PSA percentage change from baseline is as follows:
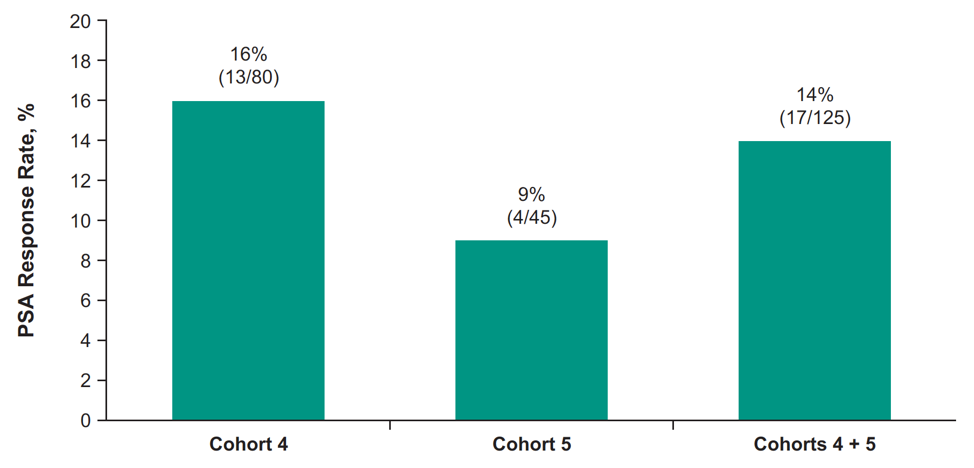

The median time to PSA progression for cohort 4 was 4.2 months (95% CI 4.1-4.4) and cohort 5 was 4.2 months (95% CI 4.2-4.4). The median rPFS was 4.2 months (95% CI 2.5-6.0) for cohort 4 and 4.4 months (95% CI 3.4-6.2) for cohort 5:
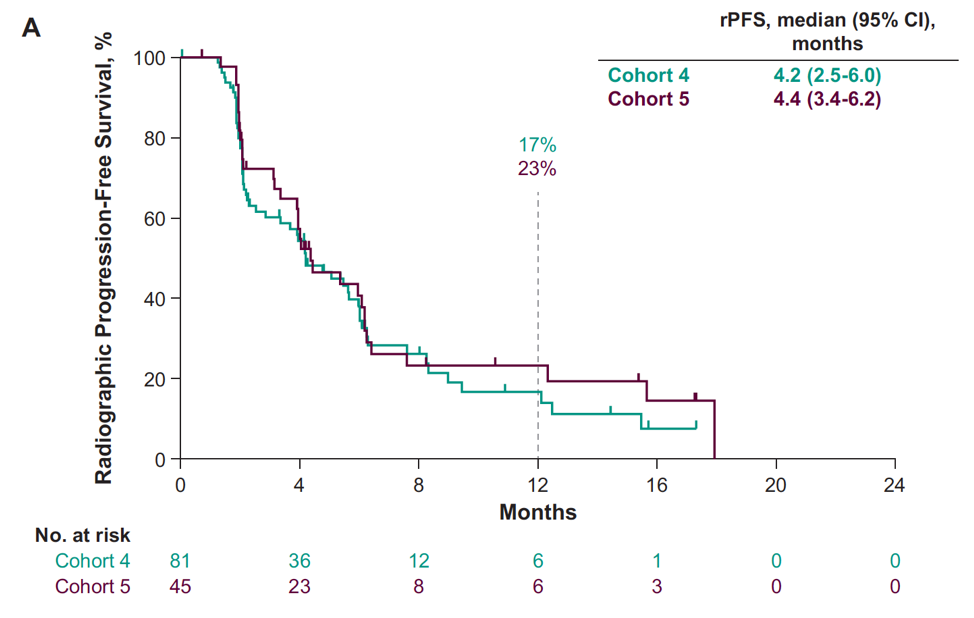
Whereas median overall survival was not reached (95% CI 15.9-NR) for cohort 4 and 18.8 months (95% CI 14.0-NR) for cohort 5:

In cohort 4, the median time to next cytotoxic chemotherapy was 11.1 months (95% CI 8.5 – NR) and time to new anticancer therapy was 9.4 months (95% CI 7.2-11.1). In cohort 5, the median time to next cytotoxic chemotherapy was 11.3 months (95% CI 9.0 – 14.5) and time to new anticancer therapy was 9.5 months (95% CI 5.9-12.1). Grade ≥3 treatment-related adverse events occurred in 26% of patients in cohort 4 and 24% in cohort 5. Two patients in cohort 4 died of immune-related adverse events (Miller Fisher syndrome and myasthenia gravis). Incidence of any grade/grade 3-4 rash (regardless of treatment relatedness) was higher than previously reported for individual agents but manageable with standard of care treatments.
Dr. Omlin concluded this presentation of KEYNOTE-199 with the following summary points:
- The addition of pembrolizumab to enzalutamide after progression on enzalutamide showed modest antitumor activity in patients with RECIST-measurable and bone-predominant mCRPC
- This combination of therapy has a manageable safety profile
- The combination is being evaluated in a randomized phase 3 study of enzalutamide with or without pembrolizumab for patients who are intolerant of or progressed on or after abiraterone acetate therapy for mCRPC without having received chemotherapy for mCRPC
Co-Authors: J.N. Graff,2 C.J. Hoimes,3 S.T. Tagawa,4 C. Hwang,5 D. Kilari,6 A.J. Ten Tije,7 R. McDermott,8 U.N. Vaishampayan,9 T. Elliott,10 W.R. Gerritsen,11 H. Wu,12 J. Kim,12 C. Schloss,12 J.S. de Bono,13 E.S. Antonarakis14
2 Medical Oncology, OHSU Knight Cancer Institute, Portland, OR, USA, 3 Medicine Division of Hematology and Oncology, Duke Cancer Institute, Durham, NC, USA, 4 Medical Oncology, VIVO, Weill Cornell Medical College, New York, NY, USA, 5 Medical Oncology, Henry Ford Health System, Detroit, MI, USA, 6 Medicine, Medical College of Wisconsin, Milwaukee, WI, USA, 7 Medical Oncology, Erasmus MC, Rotterdam, Netherlands, 8 Hematology/Oncology, Tallaght University Hospital, Dublin, Ireland, 9 Internal Medicine, Karmanos Cancer Institute, Wayne State University, Detroit, MI, USA, 10 Medical Oncology, The Christie NHS Foundation Trust, Manchester, UK, 11 Medical Oncology, Radboud University Medical Center, Nijmegen, Netherlands, 12 Medical Oncology, Merck & Co., Inc., Kenilworth, NJ, USA, 13 Medical Oncology, The Royal Marsden NHS Foundation Trust, London, UK 14 Oncology, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD, USA
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020
References:
- Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann Oncol. 2018 Aug 1;29(8):1807-1813
- Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for Treatment –Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J Clin Oncol 2020 Feb 10;38(5):395-405.
- Graff JN, et al. J Clin Oncol. 2018;36(suppl). Abstract 5047.
Related Content:
ASCO GU 2020: KEYNOTE-199: Pembrolizumab Plus Enzalutamide for Enzalutamide-Resistant mCRPC: Cohorts 4-5


