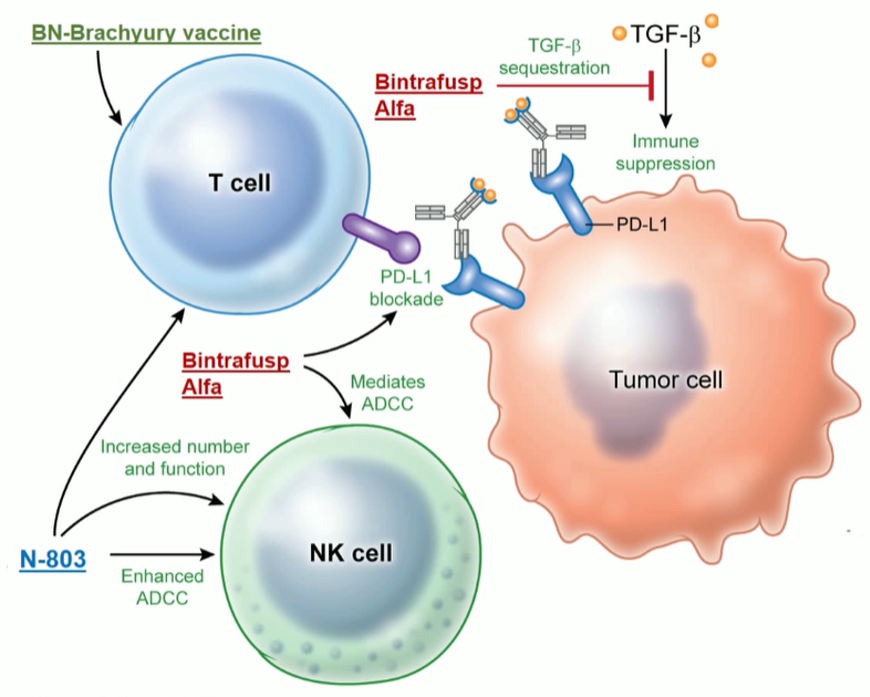
The analyzed regimen in the presented study (Figure 1) included three factors:
- BN-Brachyury vaccine (BNVax) was delivered subcutaneously. This is a poxiviral vaccine targeted to brachyury, a transcription factor involved in invasion and metastasis, with a prime and boost dosing regimen.
- Bintrafusp Alfa 1200 mg IV every two weeks, which is a bifunctional fusion protein that blocks PD-L1 and blocks TGF-BETA signaling by sequestering all isoforms
- N-8303 15 mcg/kg SC every two weeks, which is an IL-15 super-agonist complex.
Figure 1 – immunotherapy regimen:
Two study arms were chosen, as seen in Figure 2. The primary objective was to determine the response rate of each treatment regimen, defined as an objective response by RECIST v 1.1 or a prostate-specific antigen (PSA) decline of over 30% sustained for more than 21 days. Eligible patients had metastatic castration-resistant prostate cancer (mCRPC) or nonmetastatic castration-resistant prostate cancer (nmCRPC) with progressive disease defined as:
- Radiographic progression
- PSA progression (two increasing values over 1.0 ng/ml separated by one week)
Figure 2 – Study arms:

Patients were either minimally symptomatic or asymptomatic, and patients requiring standing narcotics for cancer-related pain were excluded. Patients who were given chemotherapy for CRPC must have had it more than one year ago. If chemotherapy was given for hormone-sensitive prostate cancer (HSPC), it must have been delivered more than three months before, and the patient could not have progressed on it.
Table 1 demonstrates baseline demographic and clinical data, showing that most patients were metastatic and in arm 2.1, most patients were CRPC-treatment naïve (received prior androgen deprivation therapy only [ADT]), and only five patients in arm 2.2 received no prior treatment for CRPC aside from ADT.
In arm 2.1, only one patient out of 13 (7.8%) had a PSA response while in arm 2.2, 5/9 patients (55.5%) had a PSA response (Figure 3).
Generally, all treatments were well-tolerated, with mainly grade 1-2 treatment-associated adverse events (TRAEs) (Table 2). Only three patients in each arm experienced grade 3 TRAEs, and no patient had grade 4 TRAEs.
Table 1 – Baseline demographic and clinical data:

Figure 3 – Clinical activity:
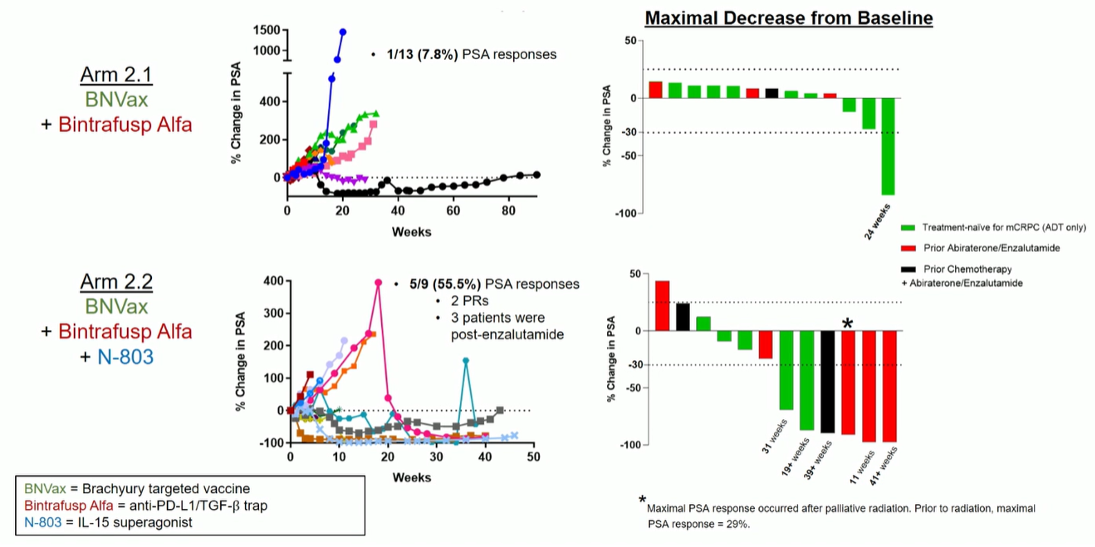
Table 2 – Treatment associated adverse events:

An interesting observation was that there was a relatively high rate of isolated adrenocorticotropic hormone (ACTH) deficiency in both treatment arms. Even more, interestingly, all four patients who had a PSA response were the ones that developed the isolated ACTH deficiency.
In summary, although the number of treated patients was quite small, the predefined clinical activity threshold in patients treated with the combination of the analyzed three factors was met in 5/9 patients (55.5%). Five patients had sustained PSA declines, and the treatment was active in patients who had previously progressed on enzalutamide. There were 3 cases of isolated ACTH deficiency, all in PSA responders. There are currently ongoing analyses exploring the association between isolated ACTH deficiency and response in this regimen. The authors plan to enroll an additional 13 patients for the study.
Presented by: Jason M. Redman, MD Internal Medicine Specialist in Washington, DC, The Johns Hopkins Hospital, Bethesda, Maryland, United States
Written by: Hanan Goldberg, MD, MSc., Assistant Professor of Urology, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020
Related Content:
Read the Invited Discussant Summary by Dr. Andrea Alimonti, MD, Institute of Oncology Research, Bellinzona, Switzerland


