(UroToday.com) At the European Society of Medical Oncology – 2020 Virtual Congress (ESMO), Dr. Andrea Alimonti began his talk discussing the 615MO abstract: “Phase 1b/2 study of VERU-111, novel, oral tubulin inhibitor, in men with metastatic castration-resistant prostate cancer (mCRPC) who failed an androgen blocking agent”, presented by Dr. Mark Markowski.
Although the therapeutic options for metastatic castrate-resistant prostate cancer (mCRPC) have been growing at an impressive rate over the last few years (Figure 1), the 5-year survival rate of mCRPC is still quite limited. The two discussed studies offer novel therapeutic options to this disease.
Figure 1 – Therapeutic options for prostate cancer:
Microtubule-targeting agents are compounds that bind to soluble and/or polymerized tubulin in the microtubules, and therefore affect microtubule function. Microtubules constitute the mitotic spindle in dividing human cells and are exquisitely sensitive to therapeutic inhibitors such as taxanes. The taxanes docetaxel and cabazitaxel have been shown to improve the survival of mCRPC patients (Figure 2).
Figure 2 – Evidence of the beneficial effect of docetaxel and cabazitaxel in prostate cancer:
In the 615MO study reported by Dr. Markowsky, clinical efficacy and safety of a novel tubulin inhibitor, the VERU-111 was presented (figure 3). Its major advantage over other tubulin inhibitors is its high affinity to both alpha and beta-tubulin. It also has good bioavailability and can be administered orally. In different preclinical models, VERU-111 has been shown to reduce tumor growth of taxane resistant prostate cancer cell lines.
Figure 3: VERU-111 mechanism:
In the presented study by Dr. Markowsky, 7 US sites were involved, and all had patients had to have one previous androgen receptor (AR) targeted therapy, and up to one taxane-based previous chemotherapy was allowed. The results showed that the adverse event profile was well-tolerated, with the most common adverse effects being diarrhea, fatigue, and vomiting. There was less severe hematological toxicity compared to other taxane-based chemotherapy.
Although the study is still ongoing, the results showed a PSA decline in 60% of patients. In summary, this study shows that the novel oral medication (VERU-111) has an impressive efficacy rate in mCRPC patients with the advantage of being delivered orally.
The second study discussed by Dr. Alimonti was the 616MO abstract: “Efficacy of BN-brachyury (BNVax) + Bintrafusp alfa (BA) + N-803 in Castration-Resistant Prostate Cancer (CRPC): Results from a Preliminary Analysis of the Quick Efficacy Seeking Trial (QuEST1), presented by Dr. Jason Redman. This study investigated an immunotherapy approach in mCRPC patients.
Prostate cancer is generally regarded as a “cold” tumor with low immunotherapeutic activity, with a low mutational tumor burden, and an immunosuppressive environment (figure 4).
Figure 4 – Prostate cancer is considered a “cold” tumor: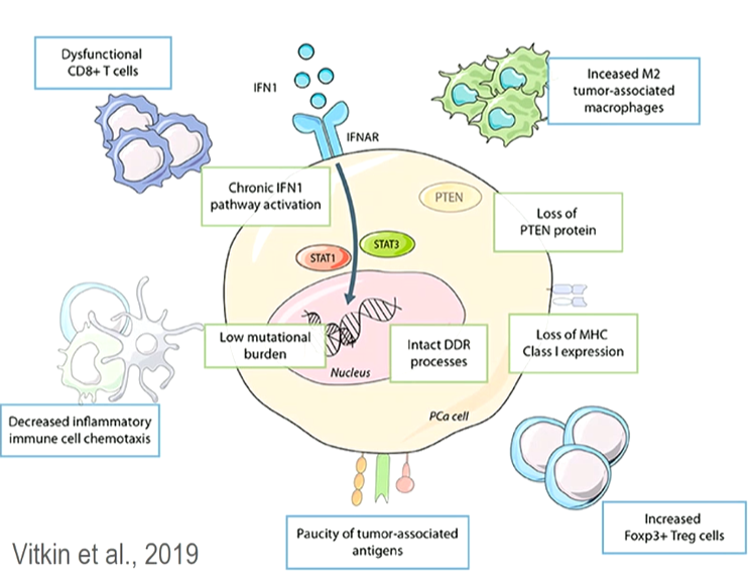
The presented study analyzed a regimen of three different immunotherapeutic factors (Figure 5):
- BN-Brachyury vaccine (BNVax) was delivered subcutaneously. This is a poxiviral vaccine targeted to brachyury, a transcription factor involved in invasion and metastasis, with a prime and boost dosing regimen.
- Bintrafusp Alfa 1200 mg IV every two weeks, which is a bifunctional fusion protein that blocks PD-L1 and blocks TGF-BETA signaling by sequestering all isoforms
- N-8303 15 mcg/kg SC every two weeks, which is an IL-15 super-agonist complex
Figure 5 – Immunotherapy regimen: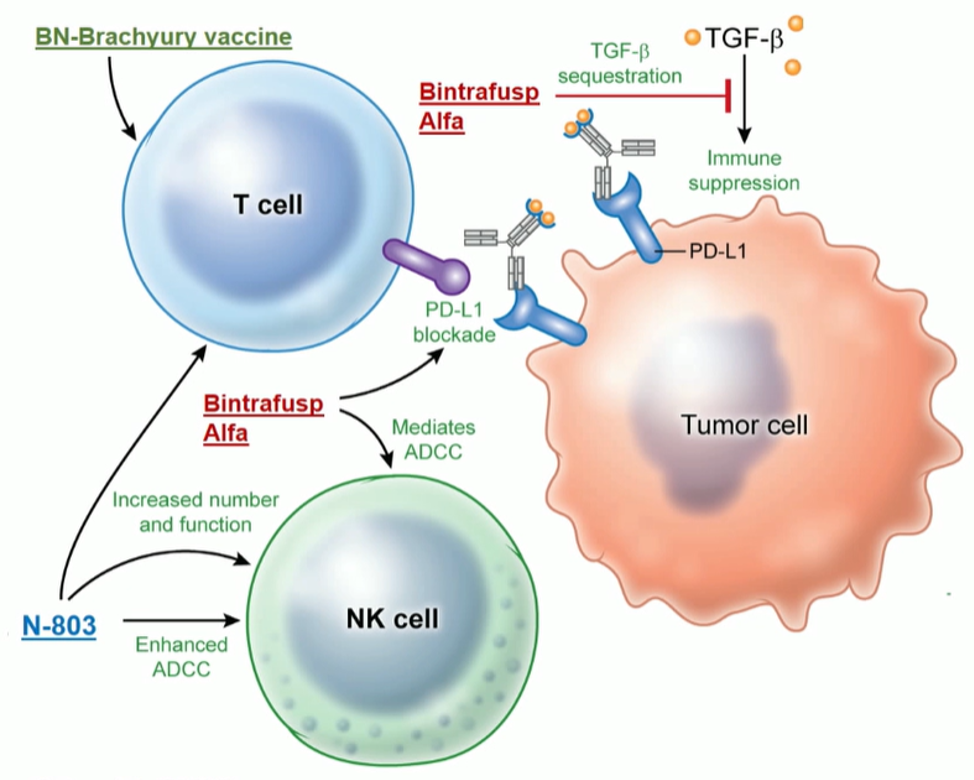
The results presented by Dr. Redman are preliminary but show a tolerable safety profile (Figure 6).
Figure 6 – Safety profile: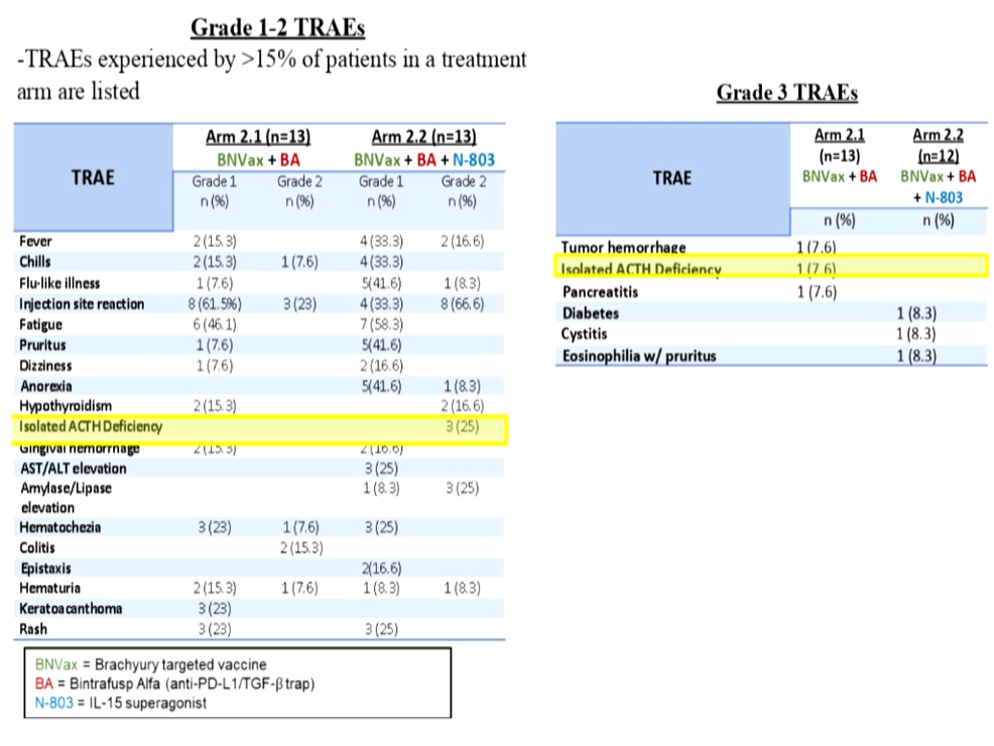
The results also showed that in arm 2.2, which consisted of all three immunotherapeutic factors, 5/9 patients (55%) had a PSA response (Figure 7). However, according to Dr. Alimonti, making any definitive conclusion from these results is hindered by the low number of patients, and more data is needed on a greater number of patients.
Figure 7 – Treatment efficacy:
Presented by: Andrea Alimonti, MD, Institute of Oncology Research, Bellinzona, Switzerland
Written by: Hanan Goldberg, MD, MSc., Assistant Professor of Urology, SUNY Upstate Medical University, Syracuse, NY, USA @GoldbergHanan at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020


