(UroToday.com) The 2022 European Society of Medical Oncology (ESMO) annual meeting featured a prostate cancer session, including a presentation by Dr. Fred Saad discussing a biomarker analysis and updated results from the Phase III PROpel trial of abiraterone and olaparib vs abiraterone and placebo as first-line therapy for patients with metastatic castration-resistant prostate cancer (mCRPC). Patients treated in the first-line mCRPC setting have a median survival of ~3 years in clinical trial settings, thus there is a need to improve patient outcomes. At the primary analysis of PROpel (NCT03732820; data cut-off July 30, 2021), abiraterone + olaparib significantly prolonged radiographic progression-free survival (rPFS) vs placebo + abiraterone in first-line mCRPC (HR 0.66, 95% CI 0.54–0.81; p <0.0001).1 Furthermore, overall survival (OS) trended towards a benefit with abiraterone + olaparib vs placebo + abiraterone (28.6% maturity; HR 0.86, 95% CI 0.66–1.12). At the 2022 ESMO meeting, Dr. Saad and colleagues reported updated overall survival and safety data from a planned OS interim analysis.
PROpel is a double-blind, placebo-controlled trial of which 796 patients were randomized 1:1 to olaparib (300 mg twice daily) or placebo, and abiraterone (1000 mg once daily) + prednisone or prednisolone (5 mg twice daily), irrespective of homologous recombination repair gene mutation status:

The primary endpoint was rPFS by investigator assessment, and OS was a key secondary endpoint. Aggregated results from tumor tissue (FoundationOne®CDx) and circulating tumour DNA (FoundationOne®Liquid CDx) tests were used to classify patients HRR mutation status.
Patients with HRR mutations, including BRCA mutations, were balanced between treatment arms. In the updated analysis, rPFS favored abiraterone + olaparib vs abiraterone + placebo in the ITT population (25.0 months vs 16.4 months; HR 0.67, 95% CI 0.56-0.81):
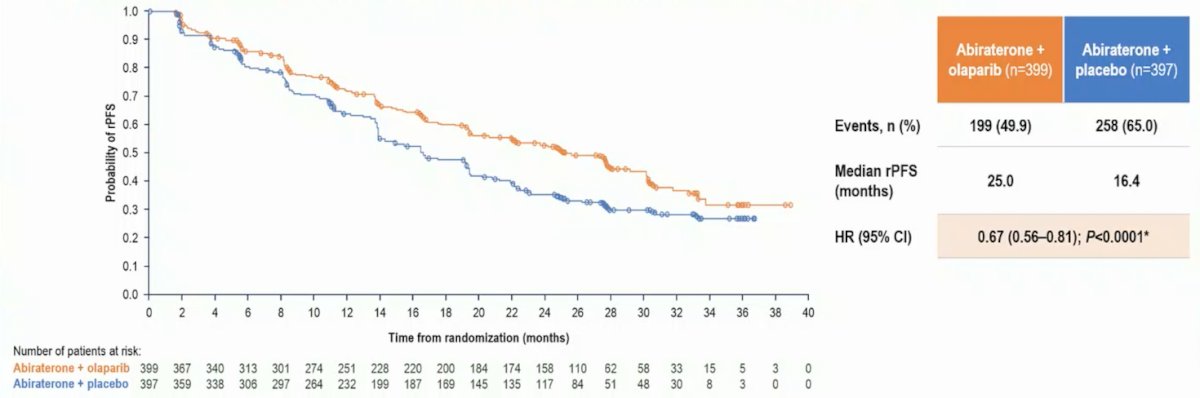
Additionally, the benefit of abiraterone + olaparib was also seen in key secondary endpoints, including time from subsequent therapy (HR 0.76, 95% CI 0.63-0.90) and PFS2 (HR 0.71, 95% CI 0.54-0.94):

Subsequent therapies were received by 39.9% of patients in the abiraterone + olaparib arm and 49.6% of patients in the abiraterone + placebo arm, with the most common being cytotoxic chemotherapy and hormonal therapy. Additionally, there was a continued trend towards improved OS with abiraterone + olaparib vs placebo + abiraterone (maturity 40.1%; HR 0.83, 95% CI 0.66–1.03), with the Kaplan-Meier curves showing clear separation between the arms at ~22 months before extensive censoring was observed:

Finally, safety and tolerability results remained stable:

Dr. Saad concluded his presentation discussing a biomarker analysis and updated results from the Phase III PROpel trial of abiraterone and olaparib vs abiraterone and placebo as first-line therapy for patients with mCRPC with the following take-home messages:
- Updated results were consistent with the initially reported results and showed a continued trend towards an OS benefit in the ITT population (HR 0.83, 95% CI 0.66-1.03)
- The safety and tolerability results were generally consistent with the primary analysis and the known profiles for abiraterone and olaparib
- Results from PROpel continue to support a superior clinical benefit with abiraterone + olaparib versus abiraterone + placebo as first-line therapy for patients with mCRPC
Presented by: Fred Saad, MD, FRCS, Professor and Chief of Urology, Director of GU Oncology, Raymond Garneau Chair in Prostate Cancer, University of Montreal Hospital Centre (CHUM), Director, Prostate Cancer Research, Institut du cancer de Montréal/CRCHUM
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.
References:
Related Content:
Discussion: ESMO 2022: Discussion of PRESTO in High-Risk Biochemically Relapsed Prostate Cancer and PROpel in mCRPC
The Efficacy and Safety of Olaparib + Abiraterone in the First-Line mCRPC Setting PROpel – Fred Saad
Conference Highlights: ESMO 2022: Biomarker Analysis and Updated Results from the Phase III PROpel Trial of Abiraterone and Olaparib vs Abiraterone and Placebo as First-Line Therapy for Patients with mCRPC


