(UroToday.com) The 2022 European Society of Medical Oncology (ESMO) annual meeting featured a prostate cancer session, including a presentation by Dr. Rahul Aggarwal discussing PRESTO, a phase 3, open-label study of androgen annihilation in patients with high-risk biochemically relapsed prostate cancer. Patients with biochemically relapsed prostate cancer following radical prostatectomy and a short PSA doubling time are at risk for distant metastases. Intermittent ADT is a standard treatment approach for biochemically recurrent prostate cancer, and a prior phase 3 study demonstrated non-inferiority of intermittent versus continuous ADT with respect to OS, with improvement in several key quality of life parameters.1 Apalutamide, an androgen receptor antagonist, and abiraterone acetate plus prednisone, prolong survival in the metastatic setting. Thus, Dr. Aggarwal and colleagues evaluated if intensification of ADT prolongs biochemical progression-free survival in biochemically relapsed prostate cancer, enabling longer treatment-free intervals within a framework of intermittent therapy.
PRESTO is a randomized phase III, open-label trial in patients with biochemically relapsed prostate cancer and PSA doubling time ≤ 9 months, without distant metastases on conventional imaging (NCT03009981). Patients were randomized 1:1:1 to receive a finite 52-week treatment course with ADT, ADT + apalutamide, or ADT + apalutamide + abiraterone acetate plus prednisone, stratified by PSA doubling time (< 3 vs 3–9 months), with post-treatment follow-up:

The primary endpoint of biochemical progression-free survival (serum PSA > 0.2 ng/mL following treatment) was compared for each experimental arm vs. control. Secondary endpoints included safety, patient-reported quality of life, time to testosterone recovery (> 50 ng/dL), metastasis-free survival (MFS) and time to castration resistance. The statistical plan for the primary endpoint is as follows:

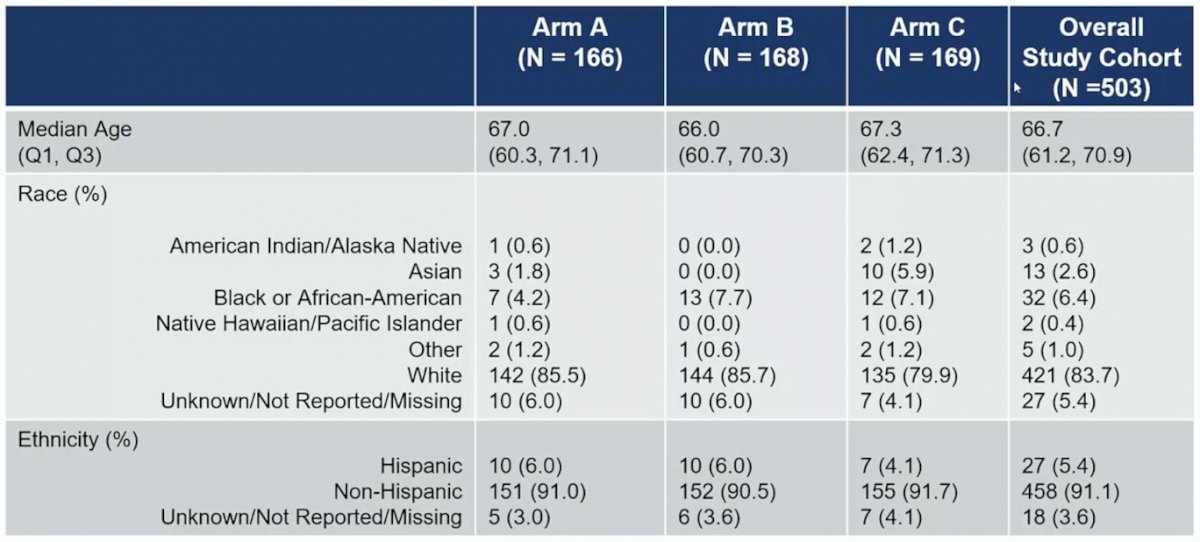
The first patient enrolled in PREST in March 2017, and in March 2020 the COVID-19 pandemic led to a mitigation plan, including remote telemedicine visits, local laboratory evaluations, drug shipment, and flexible LHRH analog dosing schedules. There were 504 patients that were randomized to ADT alone (n = 167), ADT + apalutamide (n = 168) or ADT + apalutamide + abiraterone acetate plus prednisone (n = 169). The baseline characteristics for the trial are as follows:

At the first planned interim analysis (median follow-up 21.5 months), both experimental arms significantly prolonged biochemical progression-free survival compared to the control arm: median 24.9 months for ADT + apalutamide vs 20.3 months for ADT, HR 0.52 (95% CI 0.35–0.77):

median 26.0 months for ADT + apalutamide + abiraterone acetate plus prednisone vs 20.0 months for ADT, HR 0.48 (95% CI 0.32–0.71):

PSA progression-free survival by PSA doubling time generally showed consistent results with the primary analysis:

Median time to testosterone recovery was 4.0, 3.9 and 4.8 months in ADT, ADT + apalutamide, and ADT + apalutamide + abiraterone acetate plus prednisone arms, respectively. The most common grade ≥ 2 adverse event was hypertension (19.4%, 23.4%, 30.4% in ADT, ADT + apalutamide and ADT + apalutamide + abiraterone acetate plus prednisone arms, respectively). Eight patients (1.8%) across all treatment arms stopped treatment for adverse events. Dr. Aggarwal notes that follow-up for analysis of quality of life, MFS and time to castration resistance is ongoing. The limitations of this study include (i) a PSA-based rather than metastasis-free survival endpoints, (ii) metabolic imaging (ie. fluciclovine or PSMA PET) were not required at screening (thus the truly M0 biochemically recurrent CSPC population is shrinking with stage migration), and (iii) the role of metastasis-directed therapy in oligometastatic CSPC in conjunction with ADT remains to be defined.
Dr. Aggarwal concluded his presentation discussing PRESTO, a phase 3, open-label study of androgen annihilation in patients with high-risk biochemically relapsed prostate cancer with the following take-home messages:
- More complete androgen receptor blockade with apalutamide in addition to ADT prolongs biochemical progression-free survival with a manageable safety profile, without impacting time to testosterone recovery following a finite duration of treatment
- More hypertension was seen in the abiraterone acetate plus prednisone-containing treatment arm
- Intensification of ADT should be considered in high-risk biochemically relapsed prostate cancer, but there does not appear to be a further benefit with the addition of abiraterone acetate + prednisone to apalutamide
Presented by: Rahul Aggarwal, MD, Department of Medicine, UCSF - University of California San Francisco - Parnassus Campus, San Francisco, CA
Co-Authors: G. Heller2, D. Hillman3, H. Xiao4, J. Picus5, J. Wang6, M.E. Taplin7, T. Dorff8, L.J. Appleman9, D. Weckstein10, A. Patnaik11, A.H. Bryce12, D. Shevrin13, J. Mohler14, D. Anderson15, A. Rao16, S.T. Tagawa17, A. Tan18, S. Eggener19, M.J. Morris20
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.
References:
Related Content:
Discussion: ESMO 2022: Discussion of PRESTO in High-Risk Biochemically Relapsed Prostate Cancer and PROpel in mCRPC
Conference Highlights: ESMO 2022: Biomarker Analysis and Updated Results from the Phase III PROpel Trial of Abiraterone and Olaparib vs Abiraterone and Placebo as First-Line Therapy for Patients with mCRPC


