(UroToday.com) The 2023 ESMO annual meeting included a session on urothelial carcinoma, featuring a discussant presentation by Dr. Thomas Powles. For this presentation, Dr. Powles discussed abstract “ICRA: Efficacy of paclitaxel with tremelimumab +/- durvalumab in metastatic urothelial carcinoma after progression on platinum chemotherapy and anti-PD-(L)1” by Dr. Sarah Einerhand and abstract “The Double Antibody Drug conjugate (DAD) Phase I trial: Sacituzumab govitecan plus enfortumab vedotin as ≥ second line therapy for metastatic urothelial carcinoma” by Dr. Bradley McGregor. Dr. Powles started by highlighting the rationale for the ICRA trial:
- Platinum based chemotherapy in combination with PD-(L)1 therapy has shown some antagonism. What about other chemotherapies and other immune checkpoint inhibitors?
- We have not really addressed the role of CTLA-4 with chemotherapy and tremelimumab dosing is uncertain
In ICRA, in the expansion phase (n = 12 per arm), patients received (Arm A) paclitaxel (cycle 1-6) + tremelimumab 750 mg (cycle 2-8); (Arm B) paclitaxel (cycle 1-6) plus tremelimumab 300 mg once (cycle 2) plus durvalumab 1500 mg (cycle 2-12); or (Arm C) tremelimumab 750 mg (cycle 1-7):
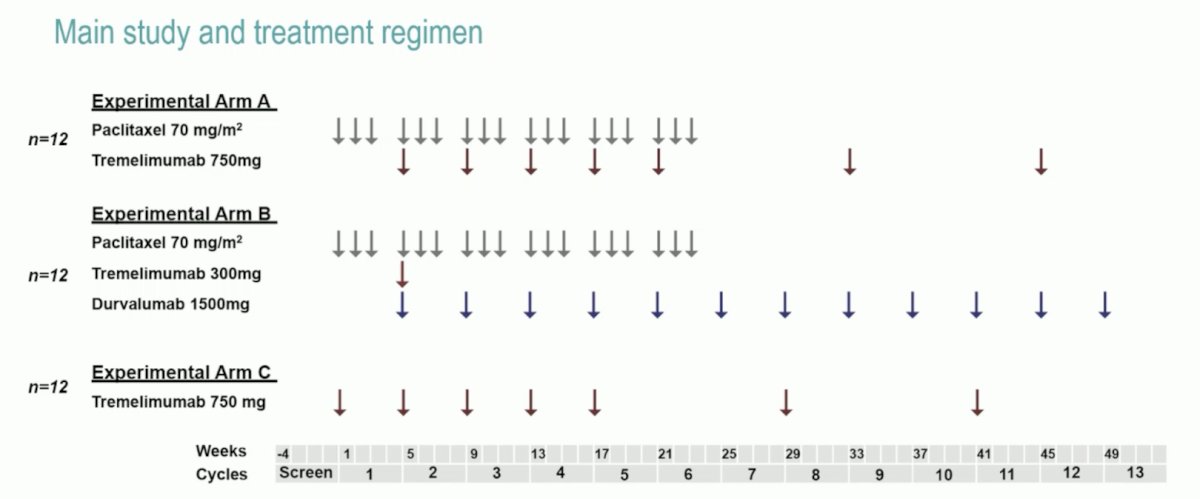
The primary endpoint was met with an ORR of 26% (88% CI 14-37%; 5/19 evaluable patients) in arm A; the ORR was 8% in arms B and C. There was tumor reduction in 50% of patients in arm A, 67% in arm B, and 17% in arm C:
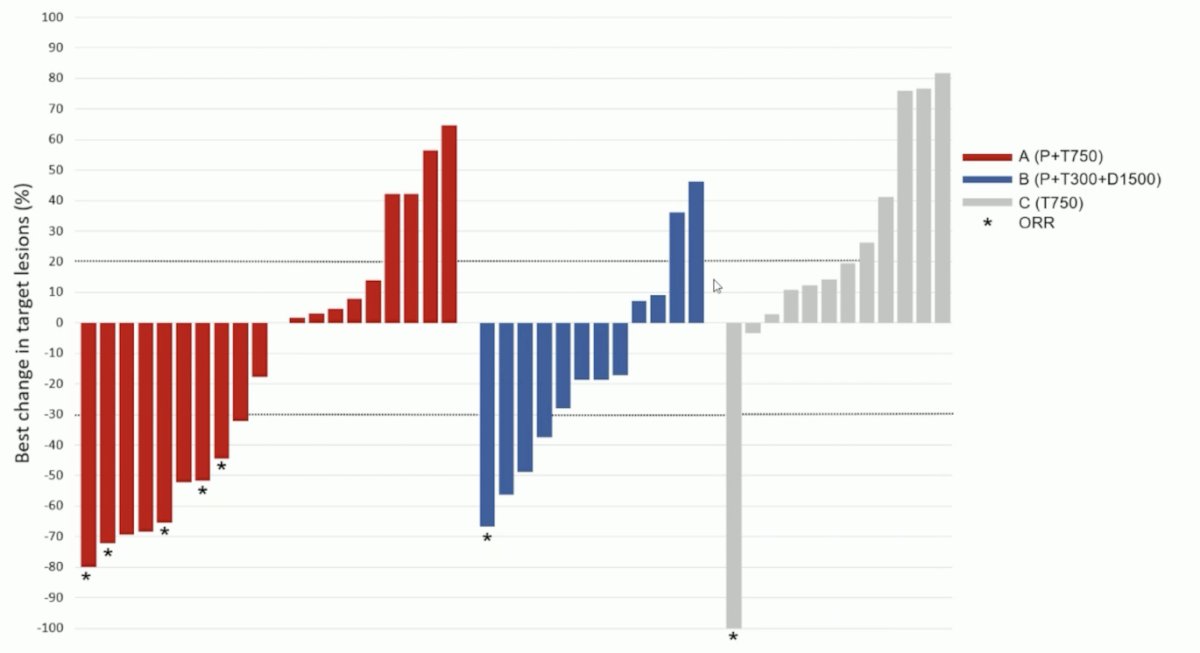
Dr. Powles notes that we have seen tremelimumab in locally advanced and metastatic urothelial carcinoma before, with previously reported objective response rates of 18.8%. Furthermore, nab-paclitaxel has previously been assessed in combination with pembrolizumab in the phase 2 PEANUT trial, showing a response rate of 39%, median PFS of 6 months, and a grade 3-4 treatment related toxicity rate of 25% [1]. Additionally, the DANUBE trial [2] combined an anti-CTLA4 (tremelimumab) + anti-PD-L1 (durvalumab) versus chemotherapy. This trial did not reach its primary endpoint of overall survival benefit, however Dr. Powles notes that tremelimumab adds about 10% to durvalumab response rate irrespective of PD-L1 status in this phase 3 trial. Currently, there are three randomized trials exploring PD-(L)1 and CTLA-4 in advanced urothelial carcinoma:
- CheckMate 901: Nivolumab + Ipilimumab as first line treatment of metastatic urothelial carcinoma
- The NILE trial: Durvalumab + tremelimumab + chemotherapy versus chemotherapy as first line treatment of metastatic urothelial carcinoma
- The VOLGA trial: Durvalumab + tremelimumab + enfortumab vedotin + cystectomy versus cystectomy alone in cisplatin ineligible operable bladder cancer
Dr. Powles then discussed the DAD phase I trial, providing the following rationale for this trial:
- Antibody drug conjugates are the most promising new class of drugs in urothelial carcinoma. Difference targets and mode of action make combinations attractive
- No one has looked at antibody drug conjugate combinations before
- Sacituzumab govitecan and enfortumab vedotin have different targets and payloads, thus toxicity and efficacy could be complimentary
In DAD, after two patients in DL1 experienced febrile neutropenia, prophylactic granulocyte stimulating factor (GCSF) was permitted, among which 18 patients received GCSF. The data for maximum tolerated dose are as follows:
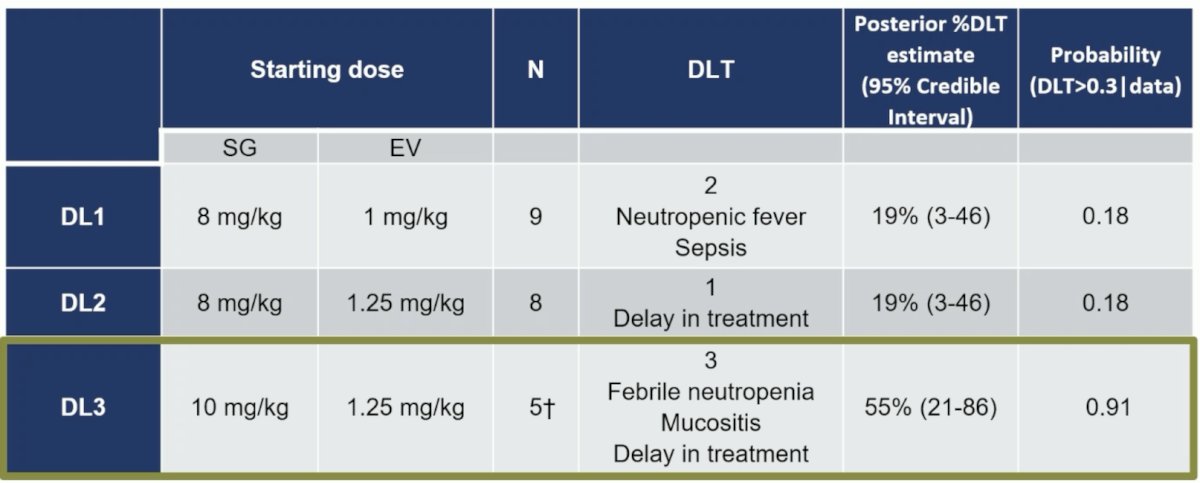
Overall, 20 of 23 patients had some degree of tumor shrinkage of their target lesion:
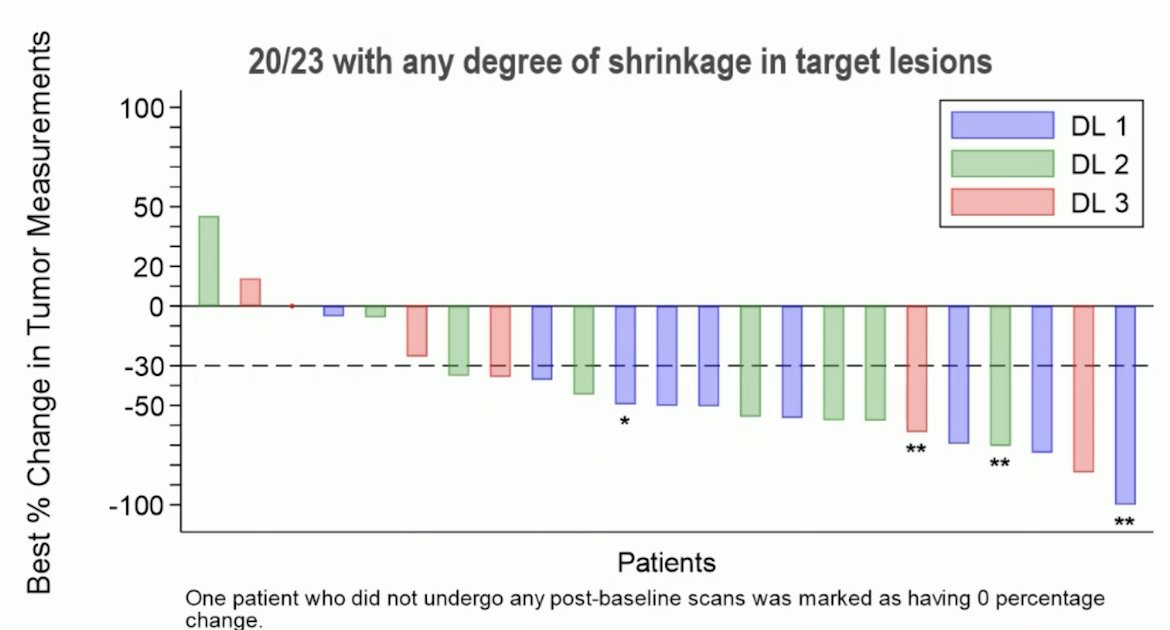
Objective response rate was 70% (15/21, 95% CI 47-87) with three complete responses, 13 partial responses, and three patients with progressive disease. The positives of this trial are: (i) the combination can be given together, (ii) there were no new safety issues, (iii) there was only one grade 5 toxicity, (iv) the adverse event profiles appear complimentary, (v) activity is better than expected in small sample size. As such, Dr. Powles feels we should take this combination forward. However, there are some issues with this trial: (i) it is not a classic 3+3 design, (ii) there were two dose limiting toxicities during dose level 1 (prior to using GCSF), (iii) some of the toxicity will appear later, (iv) there was intra-patient dose variability, (v) what you see may not be what you get. Dr. Powles states that he likes this combination, but we still have some dosing issues.
Dr. Powles feels like we may have an opportunity to go outside the disease and look at other malignancies for potential antibody drug conjugates and targets. For example, there is a phase 2 study of T-DXd in patients with HER-2 expressing solid tumors in the second line and later population. Second, I-DXd (a B7-H3 directed antibody drug conjugate) is being assessed in refractory small cell lung cancer as a subgroup analysis of a phase I/II trial. Picking the combination to broaden targeting and reduce dose limiting toxicity seems wise, according to Dr. Powles:
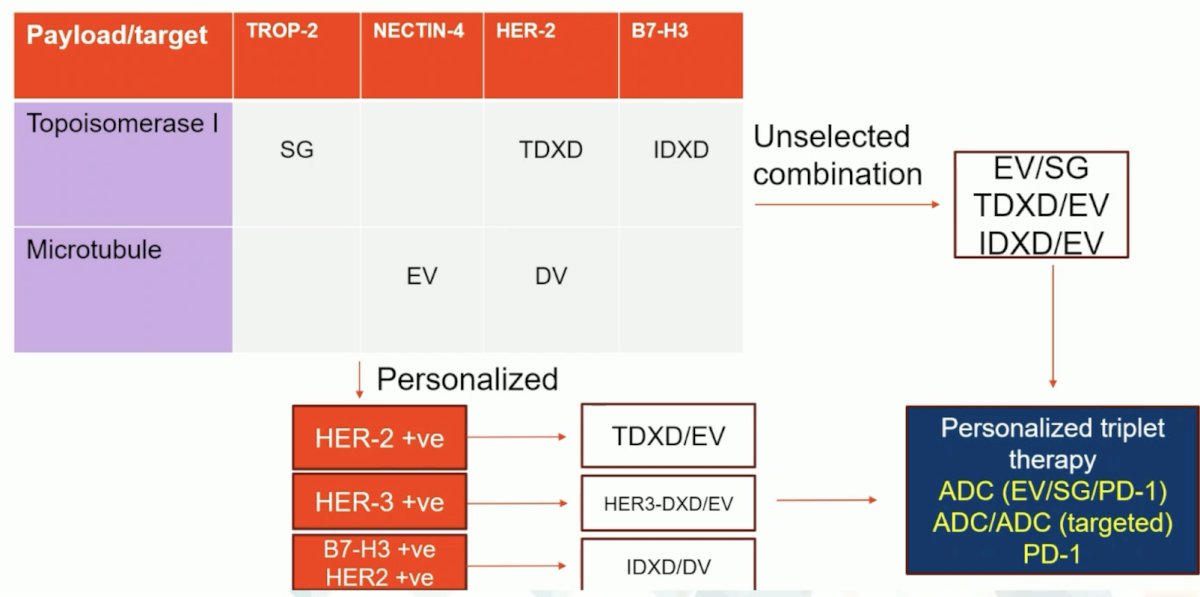
Initial results from VOLGA have shown downstaging in 9/17 patients and pathologic complete response in 6/17 patients. Hypothetically, a first line triplet therapy trial design may look as follows:
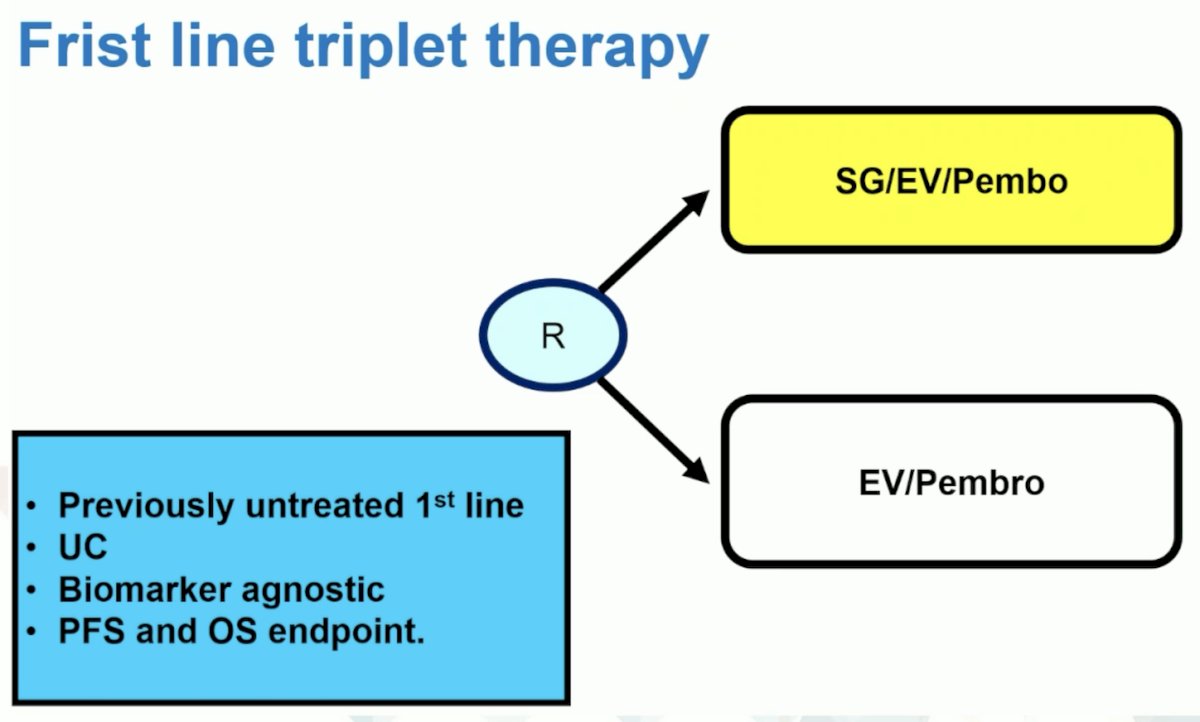
However, if we want to get more nuanced and look at other targets/antibody drug conjugates, perhaps a first line triplet therapy design looks as follows:
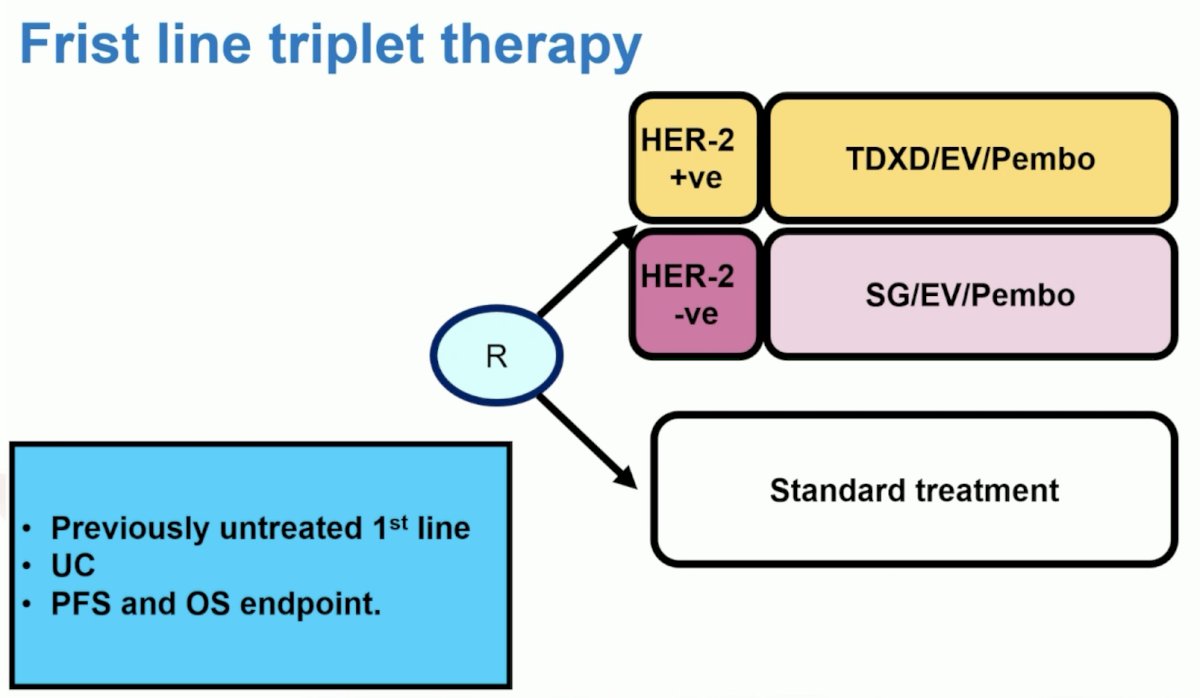
Dr. Powles concluded his discussant presentation with the following take-home points:
- The CTLA-4 story has been mixed, however there are studies targeting CTLA-4 ongoing (NILE and VOLGA)
- It looks like CTLA-4 dose may be relevant, but we may never really know
- Combinations with chemotherapy and immune therapy are complex and many questions remain in urothelial carcinoma regimens
- Antibody drug conjugate combinations is a big question in urothelial carcinoma and beyond
Presented by: Thomas B. Powles, MBBS, MRCP, MD, Cancer Research UK Barts Centre, London, United Kingdom
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Giannatempo P, Raggi D, Marandino L, et al. Pembrolizumab and nap-paclitaxel as salvage therapy for platinum-treated, locally advanced or metastatic urothelial carcinoma: Interim results of the open-label, single-arm, phase II PEANUT study. Ann Oncol. 2020;31(12):1764-1772.
- Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced urothelial carcinoma (DANUBE): A randomized, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574-1588.
ESMO 2023: ICRA: Efficacy of Paclitaxel with Tremelimumab +/- Durvalumab in Metastatic Urothelial Carcinoma After Progression on Platinum Chemotherapy and Anti-PD-(L)1
ESMO 2023: The Double Antibody Drug Conjugate (DAD) Phase I Trial: Sacituzumab Govitecan plus Enfortumab Vedotin as ≥ Second Line Therapy for Metastatic Urothelial Carcinoma


