(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a non-prostate, genitourinary tumors mini oral session. Dr. Joost Boormans delivered the discussant for the preceding two late-breaking, oral abstract presentations:
- LBA104 - First safety and efficacy results of the TAR-210 erdafitinib intravesical delivery system in patients with non–muscle-invasive bladder cancer (NMIBC) with select FGFR alterations
- LBA105 - Results From SunRISe-1 in Patients (Pts) With Bacillus Calmette–Guérin (BCG)-Unresponsive High-Risk Non–Muscle-Invasive Bladder Cancer (HR NMIBC) Receiving TAR-200 Monotherapy
Dr. Boormans began by reiterating the current definition of BCG-unresponsive disease, defined by at least one of the following:
- Persistent or recurrent CIS alone or with recurrent Ta/T1 disease within 12 months of completion of adequate BCG therapy
- Recurrent high-grade Ta/T1 disease within 6 months of completion of adequate BCG therapy
- T1 high-grade disease at the first evaluation following an induction BCG course
In this context, adequate BCG therapy is defined as at least one of the following:
- At least five of six doses of an initial induction course plus at least two of three doses of maintenance therapy
- At least five of six doses of an initial induction course plus at least two of six doses of a second induction course
What is the unmet need in BCG-unresponsive NMIBC? We need to weigh the risks of disease progression versus recurrence. The 1-year risk of progression is estimated at about 15%, with progressors having poor outcomes. Conversely, the 1-year risk of recurrence is 40 – 60%, which creates a significant clinical challenge in this patient population. BCG unresponsive CIS is difficult to treat in the long-term.
The current issues with emerging novel therapies for BCG-unresponsive NMIBC remain numerous:
- Different primary endpoints chosen for different trials, limiting comparisons
- Not all trials mandate biopsies to assess disease response
- Use of single arm phase II trial design with no comparator group
- Numerous visits required during the 1st year, ranging between 4 and 18
- Different toxicity profiles with intravesical versus intravenous agents
Dr. Boormans next went on to discuss currently available intravesical and intravenous drugs for patients with BCG unresponsive NMIBC.
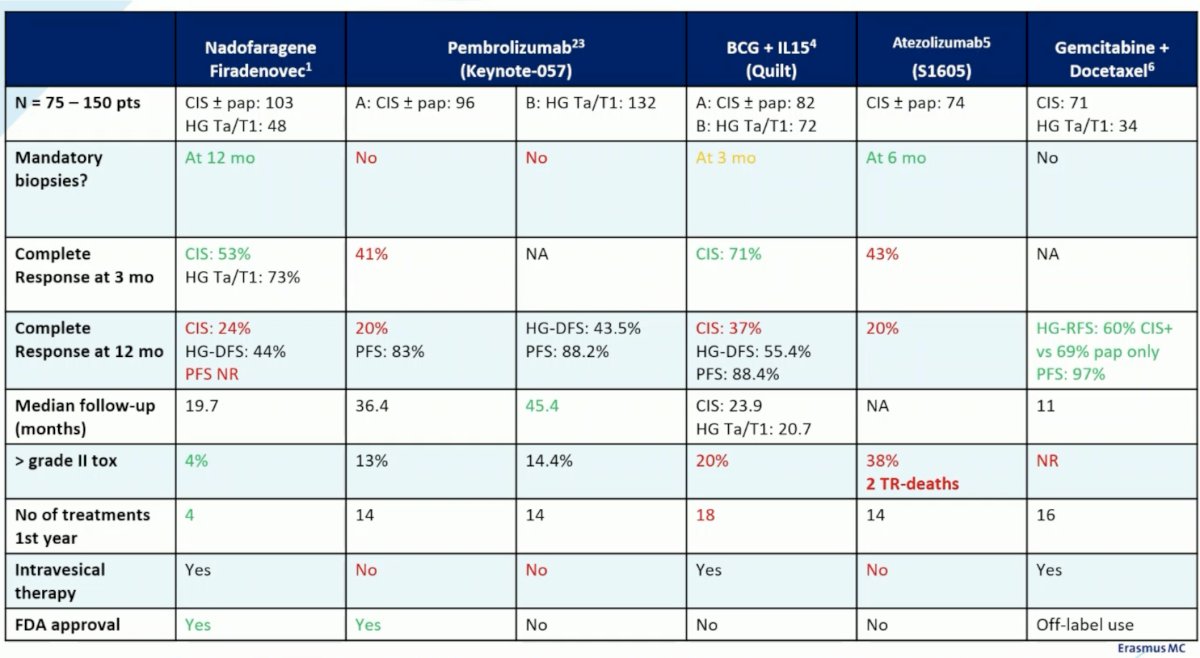
These trials classically included patients with both CIS (+/- papillary disease), with the Nadofarogene Firadenovec trial,1 KEYNOTE-057 Cohort B,2 QUILT,3 and the retrospective gemcitabine/docetaxel series4 including patients with pure papillary disease. Another important issue is the variability in mandated biopsies between these studies. Biopsies were mandated for the Nadofarogene Firadenovec trial (12 months), QUILT (3 months), and S1605 (6 months) only. Dr. Boormans argued that biopsies should be mandated in this setting to ‘faithfully’ evaluate the response rate, although he argued that 3 months as in the QUILT trial may be too soon.
From an efficacy standpoint, the complete response rate at 3 months appears to range between 41% and 71% (highest in QUILT), although we do note a steep drop off by 12 months with response rates almost halving to 20 – 40%. The longest follow-up for these studies remains the longest for pembrolizumab (KEYNOTE-057), with a median reported follow-up of 45.4 months.
We also need to factor in the number of treatment visits required in the 1st year. While Nadofarogene Firadenovec only requires 4 visits in the first year, BCG + IL-15 in the QUILT trial required 18 visits, with the other treatments ranging between 14 and 16 visits in the first year.
In light of the data for TAR-210 (intravesical erdafitinib) and TAR-200 (intravesical gemcitabine), Dr. Boormans framed the results from these studies in the context of the currently FDA approved agents in this space: Nadofarogene Firadenovec and pembrolizumab. From an eligibility standpoint, the studies of TAR-210 included only patients with papillary HG Ta/T1 disease (n=16), whereas that of TAR-200 Cohort 2 included only those with CIS disease +/- papillary (n=54). Biopsies were not mandated in SunRISe-1 (TAR-200), and it is unclear whether they were mandated for TAR-210. The complete response rates at 3 months for both were impressive at 77 – 82%, with 12-months data pending. Grade 3+ adverse events occurred in 1/6 patients receiving TAR-210 and 17% of those receiving TAR-200. The number of treatments required in the 1st year are 4 and 10 for TAR-210 and TAR-200, respectively and both are administered intravesically.
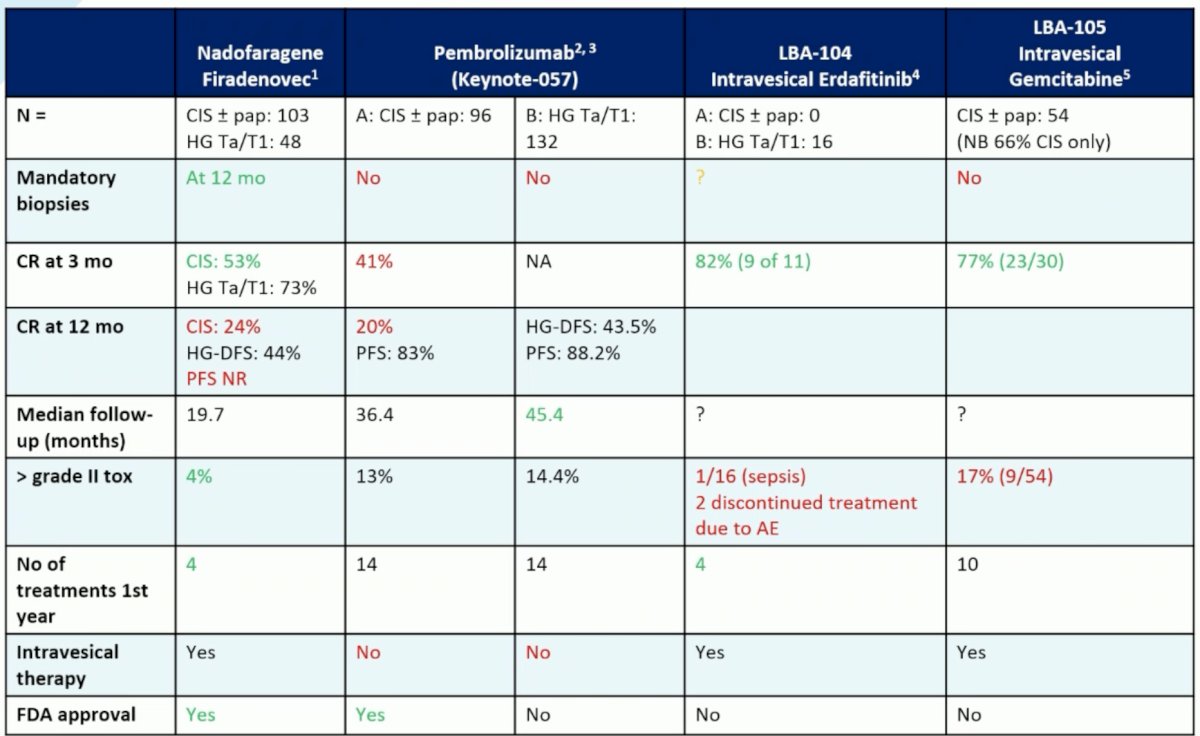
Dr. Boormans concluded his presentation with the following take home messages:
- The populations in the TAR-210 and SunRISe-1 (TAR-220) trials were different:
- FGFR alterations required for TAR-210 (erdafitinib)
- Papillary only with TAR-210 versus CIS +/- papillary for TAR-200
- Patients receiving TAR-210 were BCG-exposed versus BCG-unresponsive with TAR-200
- The studies have small numbers and short follow-up
- The early 3 months complete response rates are promising
- In the 1st year, 4 treatments are required for TAR-210 versus 10 with TAR-200
- Local side effects should not be neglected
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2020 Nov 27:S1470-2045(20)30540-4.
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicenter, phase 2 study. Lancet Oncol. 2021 Jul;22(7):919-930.
- Chamie K, Chang SS, Kramolowsky E, et al. IL-15 Superagonist NAI in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer. NEJM Evid 2022; 2(1)
- Steinberg RL, Thomas LJ, Brooks N, et al. Multi-Institution Evaluation of Sequential Gemcitabine and Docetaxel as Rescue Therapy for Nonmuscle Invasive Bladder Cancer. J Urol 2020;203(5):902-909.
ESMO 2023: TAR-210 Erdafitinib Intravesical Delivery System in Patients with Non–Muscle-Invasive Bladder Cancer with Select FGFR Alterations: First Safety and Efficacy Results
ESMO 2023: SunRISe-1: TAR-200 Monotherapy in Patients With BCG Unresponsive High-Risk Non–Muscle-Invasive Bladder Cancer (HR NMIBC)


