(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a non-prostate, genitourinary tumors mini oral session. Dr. Deborah Mukherji delivered the discussant for the two preceding oral abstract sessions:
- MEDI5752 (volrustomig), a novel PD-1/CTLA-4 bispecific antibody, in the first line treatment of 65 patients with advanced clear cell renal
- Phase II study of avelumab (Ave) plus intermittent axitinib in previously untreated patients with metastatic renal cell carcinoma: The TIDE-A study.
The currently available systemic therapy options for the 1st line treatment of advanced clear cell RCC are summarized below: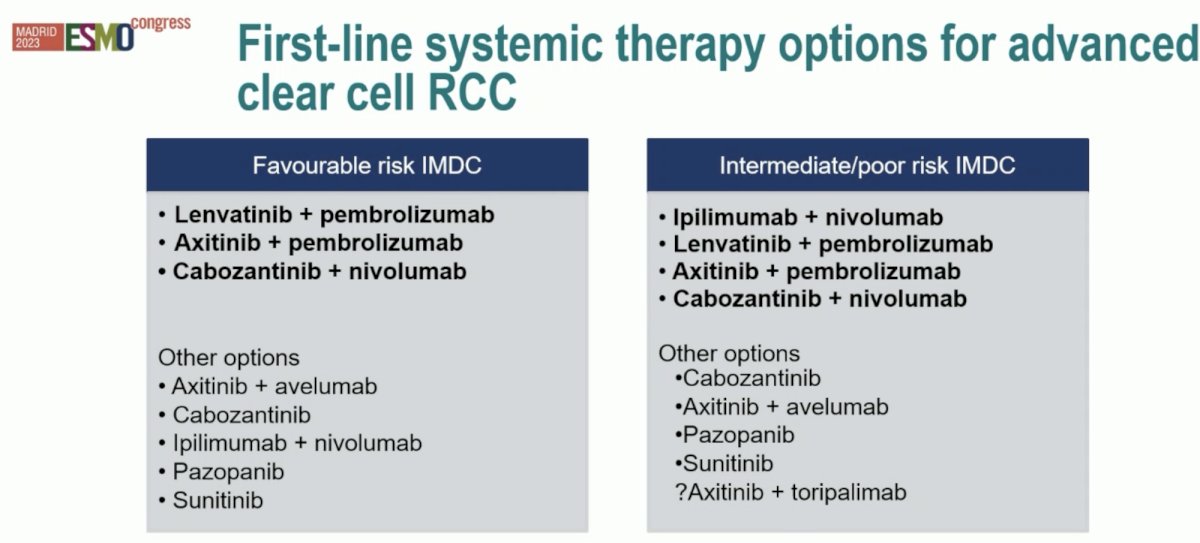
When we think about future developments with novel agents and/or combinations in this space, it is always critical to consider what our patients are looking for. In a 2022 survey of patients with metastatic RCC, it was clear that a treatment with a high chance of eliminating all evidence of disease (i.e., a complete response) was the patients’ top priority. But also important to these patients was ‘the ability to go off therapy’. Dr. Mukherji noted that these two trials of volrustomig and TIDE-A address these two issues.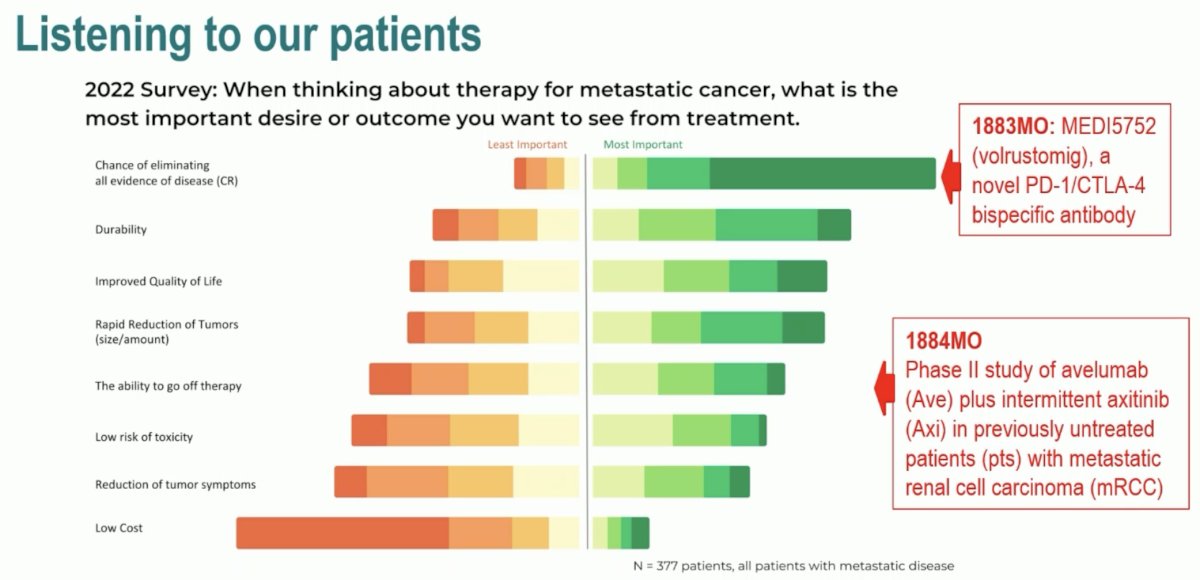
When considering complete response rates and treatment durability, the patients’ top two priorities, can we improve on these outcomes compared to currently available PD-1/CTLA-4 combinations (nivolumab + ipilimumab)? In the CheckMate214 trial, >53% of patients were still alive following nivolumab + ipilimumab.1
In the COSMIC 313 trial which evaluated the triplet therapy combination of cabozantinib addition to nivolumab + ipilimumab, there was evidence of a PFS (HR: 0.63. 95% CI: 0.47 – 0.85) and ORR improvement (45% versus 35%) in the IMDC intermediate risk subgroup, there was no increase in the complete response rate and OS data remains immature. Significantly, toxicity was a major problem, with 90% dose modifications and 45% at least one treatment discontinuation in the triplet arm.2
But what does a complete response mean in terms of durability? It appears that nearly 75% of mRCC patients are asking for a response that is durable for up to 5 years. Can we reach this response durability without dose-limiting toxicity?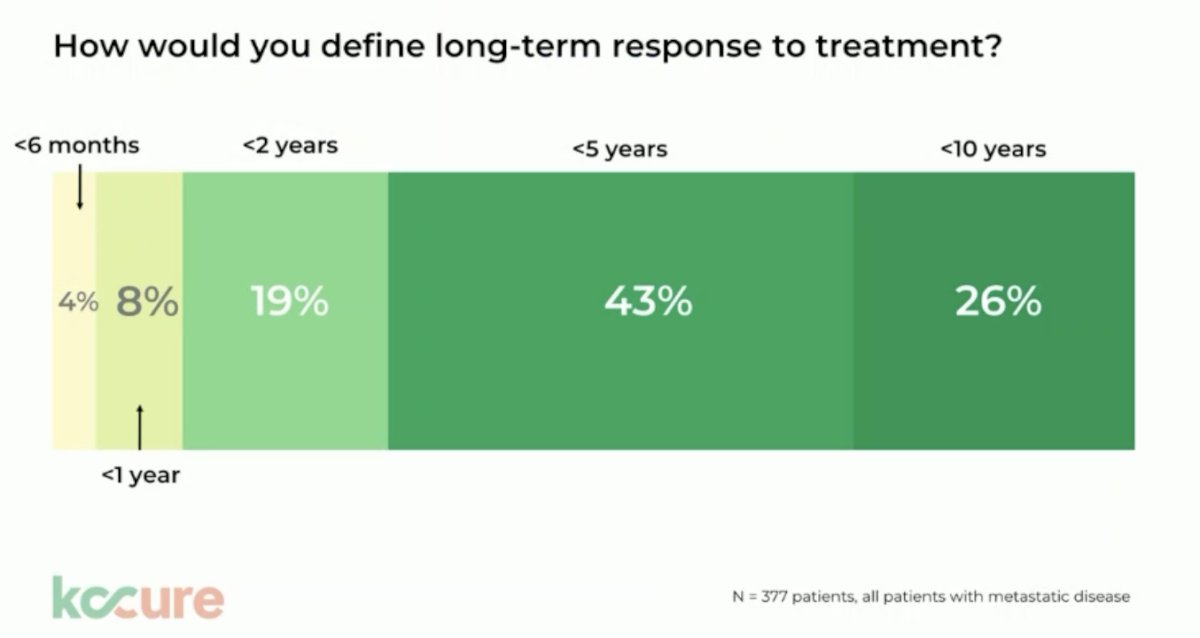
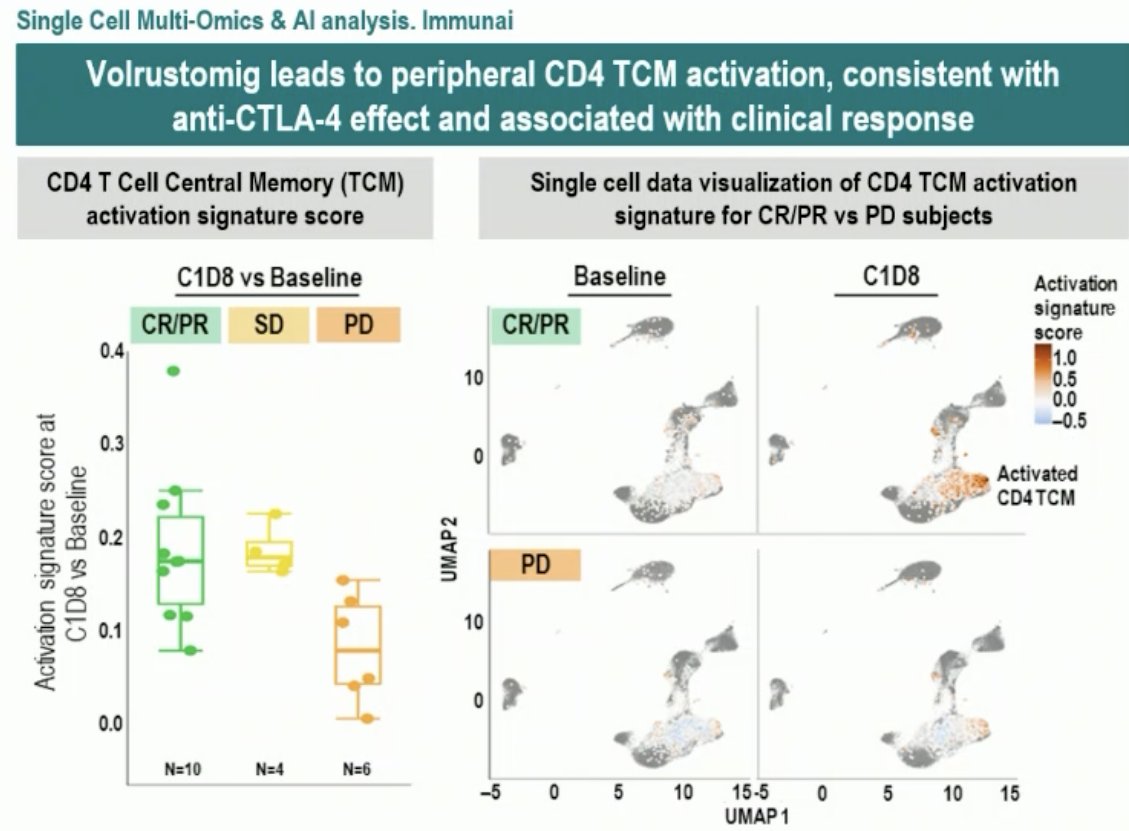
Volrustomig (MEDI5752) is a monovalent bi-specific monoclonal antibody binding PD-1 and CTLA-4. It was first evaluated in a human study at 2.25 – 2,500 mg IV doses every 3 weeks in advanced solid tumors (NCT03530397) and was shown to lead to a dose-dependent increase in T-cell proliferation with durable responses of 17.5 months. In mRCC dose expansion cohorts, durable responses have been demonstrated, with the 750 mg dose having similar efficacy to the 1,500 mg dose with improved tolerability.3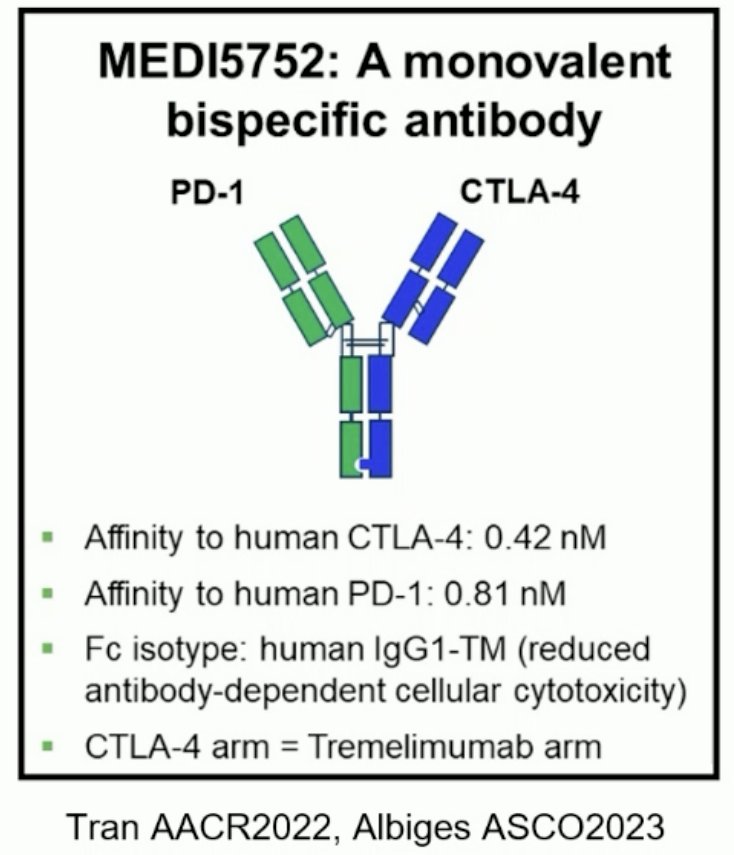
Theoretical advantages of bi-specific PD-1/CTLA-4 antibodies are as follows:
- Preferential binding to CLTA-4 on PD-1 activated dual positive tumor T-cells potentially limiting toxicity to organs
- Cooperative binding leads to increased pro-inflammatory cytokine expression from activated T-cells (associated with clinical response)
- Increased internalization and degradation of the PDL-1 receptor may lead to more durable responses
Volrustomig at either the 750 mg or 500 mg dosages does not quite reach the patient-desired duration of therapy intervals of 5 years, with median durations of response to date of 17 and 11.5 months, respectively.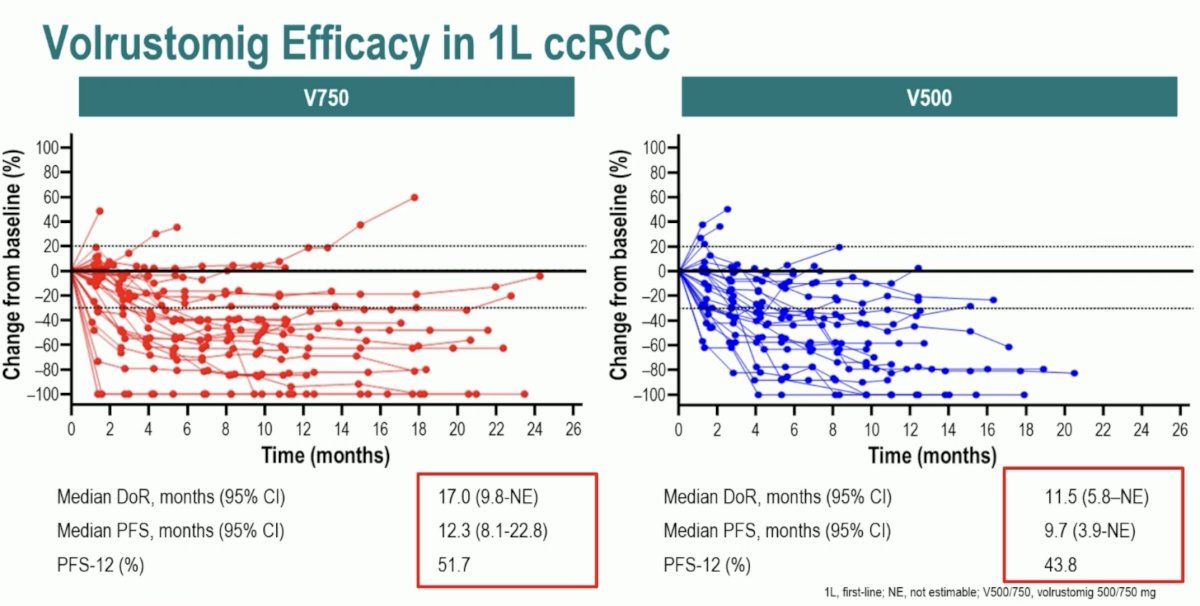
With regards to this study, Dr. Mukherji concluded as follows:
- Volrustomig is an exciting new strategy to target PD-1 and CTLA-4 with a bi-specific antibody
- It shows promising efficacy in mRCC, with careful evaluation of dose, response, and toxicity trade off needing to be evaluated in combination with TKIs
- It is aligned with patient and physician wishes to improve complete and durable responses, but not quite hitting the desired thresholds yet
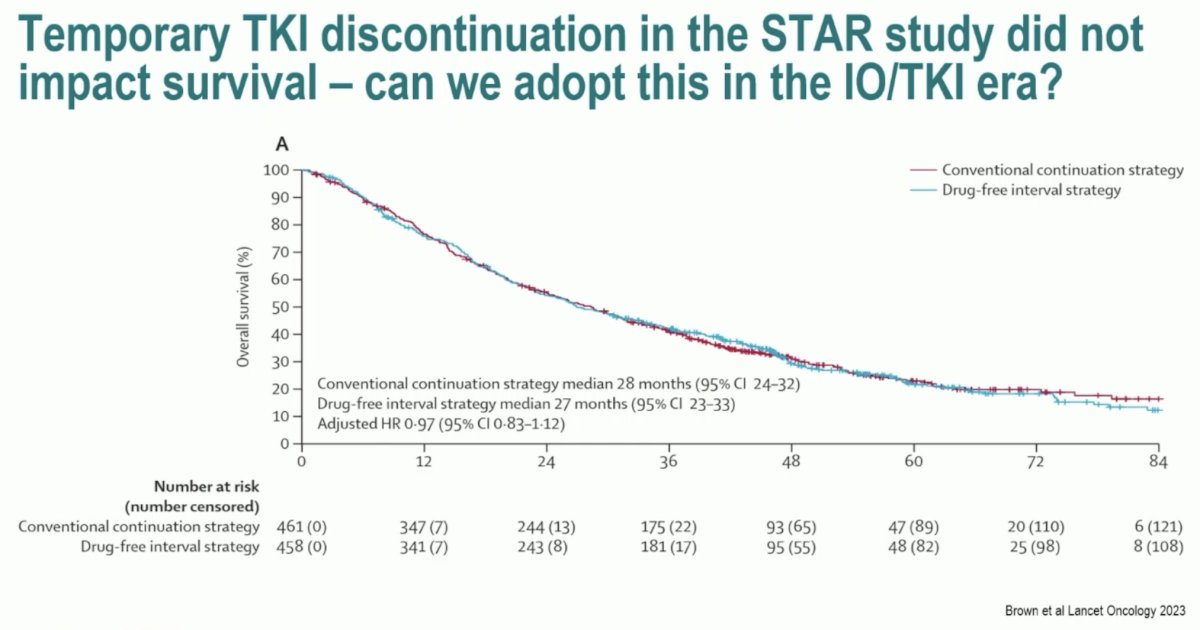
The TIDE-A study addressed patients’ desire to potentially receive a treatment that could be interrupted after an initial response. In the STAR trial, temporary TKI discontinuation did not impact OS.4 Can this paradigm be transferred to the IO/TKI era?
This phase II trial included 75 patients with metastatic ccRCC with no primary tumor, no bulky/symptomatic disease, and no liver metastases. These patients were planned for 36 weeks of avelumab 800 mg IV every 2 weeks + avelumab 5 mg oral twice daily. A tumor evaluation was performed at 36 weeks:
- In patients with a complete or partial response, the axitinib was discontinued and avelumab continued at same dose every 2 weeks until evidence of progressive disease, in which case dual therapy was re-initiated
- Patients with stable disease would continue the combination until evidence of progressive disease
- Those with progressive disease would naturally discontinue this therapy
The primary endpoint was the rate of patients free of progression after 8 weeks from axitinib discontinuation.
Notably, this trial selected patients with good prognostic features. It aimed to show that >48% of patients who were able to discontinue axitinib at week 36 were still progression-free 8 weeks later on maintenance avelumab. This trial exceeded expectations with >72% remaining progression-free 8 weeks following axitinib discontinuation.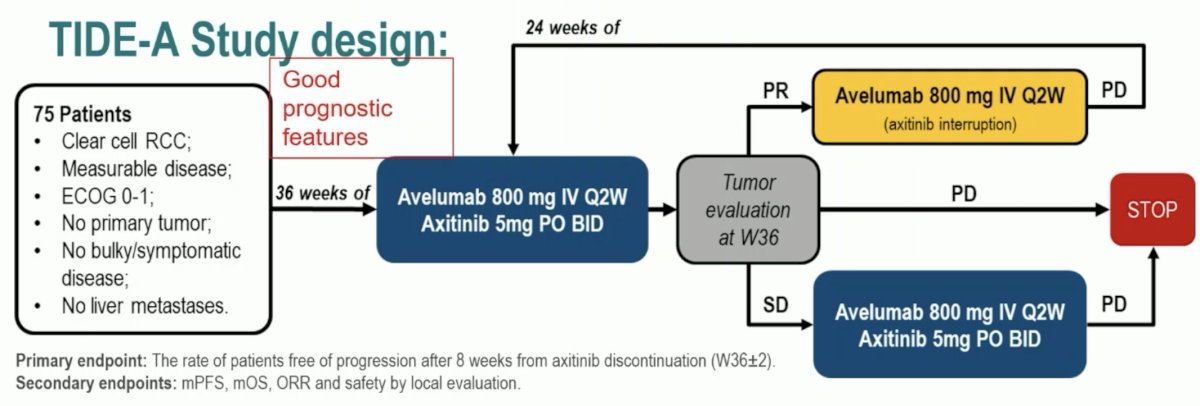
Among the 29 responders who interrupted axitinib treatment, 11/29 were still receiving maintenance therapy. Of the 18 patients progressing following avelumab maintenance, 17 re-started axitinib. 8/17 responded to this re-challenge, but 6/17 had evidence of continued progression.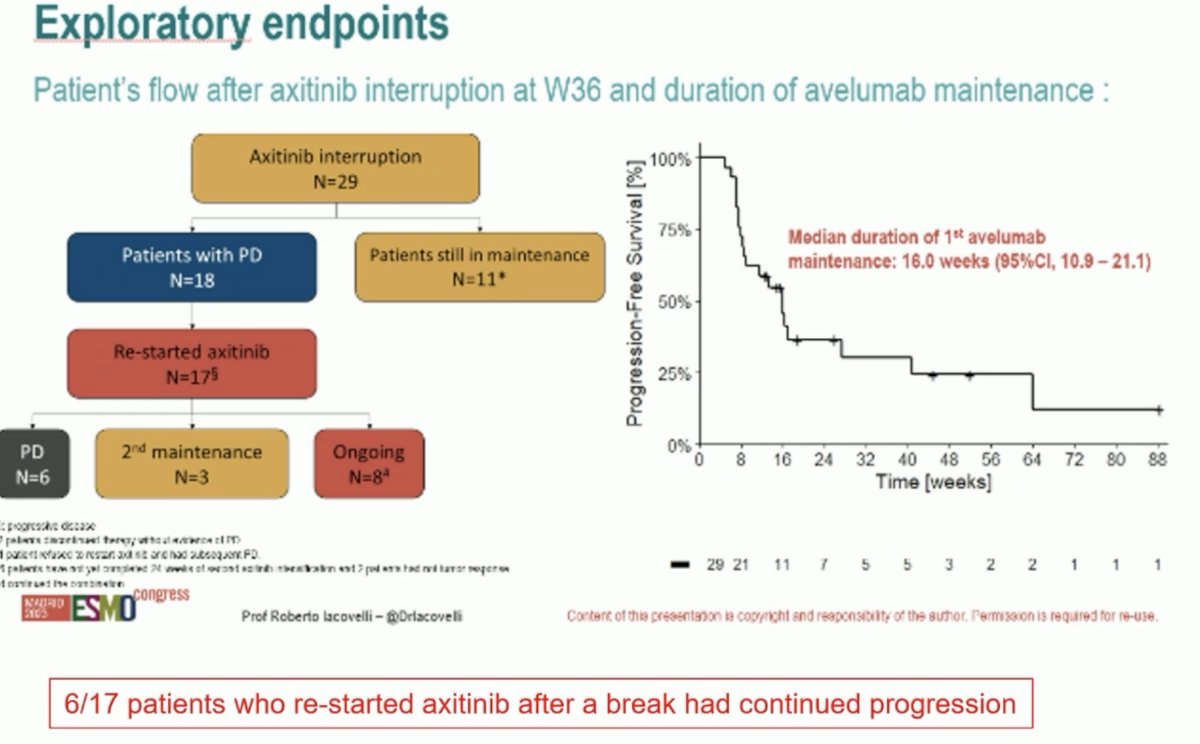
Dr. Mukherji concluded that:
- TIDE-A is a pragmatic study showing that TKI de-escalation while maintaining immunotherapy is feasible for patients responding to first-line avelumab + axitinib for mRCC.
- De-escalation strategies have the potential to spare toxicity and cost (however not top of the list for our patients surveyed)
- Patient selection is key - we are using response as a biomarker but can we use more accurate tools in the future to guide treatment selection and potentially de-escalation? (Circulating biomarkers)
Presented by: Deborah Mukherji, MBBS, FRCP, Medical Oncology Consultant, Clemenceau Medical Center, Dubai, United Arab Emirates
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023
References:- Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020;5(6):e001079.
- Choueiri TK, Powles T, Albiges L, et al. Cabozantinib plus Nivolumab and Ipilimumab in Renal-Cell Carcinoma. N Engl J Med. 2023;388:1767-1778.
- Albiges L, Rodriguez LM, Kim S, et al. Safety and clinical activity of MEDI5752, a PD-1/CTLA-4 bispecific checkpoint inhibitor, as monotherapy in patients (pts) with advanced renal cell carcinoma (RCC): Preliminary results from an FTIH trial. J Clin Oncol. 2022;40(Suppl 16):107.
- Brown JE, Royle K, Gregory W, et al. Temporary treatment cessation versus continuation of first-line tyrosine kinase inhibitor in patients with advanced clear cell renal cell carcinoma (STAR): an open-label, non-inferiority, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2023;24(3):213-227.
ESMO 2023: MEDI5752 (Volrustomig), a Novel PD-1/CTLA-4 Bispecific Antibody, in the First-Line Treatment of 65 Patients with Advanced Clear Cell Renal Cell Carcinoma
ESMO 2023: TIDE-A: Phase II Study of Avelumab plus Intermittent Axitinib in Previously Untreated Patients with mRCC


