(UroToday.com) The 2023 ESMO annual meeting included a session on prostate cancer, featuring a discussant presentation by Dr. Himisha Beltran. Dr. Beltran discussed four abstracts including “Pembrolizumab + enzalutamide for patients with mCRPC: randomized double-blind phase 3 KEYNOTE-641 study” by Dr. Julie Graff, “Pembrolizumab + enzalutamide and ADT for patients with mHSPC: randomized double-blind phase 3 KEYNOTE-991 study” by Dr. Christian Gratzke, “Niraparib with abiraterone acetate plus prednisone as first-line therapy in patients with mCRPC and HRR gene alterations: Three-year update and final analysis of MAGNITUDE” by Dr. Kim Chi, and “Androgen receptor pathway inhibitors or taxanes for patients with mCRPC: A direct comparison in ProBio, a randomized, outcome-adaptive, biomarker-driven platform trial” by Dr. Henrik Grönberg.
For the two KEYNOTE trials assessing pembrolizumab + enzalutamide, it is important to try and assess what we can learn from these negative trials. To do so, we need to take a step back and determine what is different about prostate cancer. Importantly, there are few mutations in prostate cancer and fewer neoantigens. Second, there is a predominance of immunosuppressive cells (regulatory T cells, M2 macrophages) and upregulation of immune checkpoints in microenvironments (ie. PDL1, VISTA). Third, prostate cancer is a cold tumor, with less immune infiltration, however it can be modulated by therapies such as ADT.
The rationale for combining pembrolizumab with enzalutamide is that enzalutamide increases the number of circulating PD-L1/L2+ dendritic cells and PD1+ T cell, activates IFN-gamma, and decreases immunosuppressive cells (ie. myeloid-derived suppressor cells). Furthermore, PD-L1 is expressed in enzalutamide resistant tumors. In a phase 2 trial, 5 of 28 patients had a PSA50 response and 3 of 12 patients with measurable disease at baseline achieved an objective response. Of 5 responders, 2 continued with durable response at 39.3 and 37.8 months, and median PSA-PFS was 3.8 months, time to subsequent therapy was 7.2 months, and median overall survival was 21.9 months vs 41.7 months in non-responders.
In the negative IMbassador 250 trial,1 759 men with mCRPC whose disease progressed on abiraterone were randomized to atezolizumab with enzalutamide versus enzalutamide alone. The intervention arm did not meet the primary endpoint of improved OS (sHR 1.12, 95% CI 0.91-1.37). However, there were some correlatives, including longer PFS with high PD-L1 IC2/3, CD8 expression, and establishing immune gene signatures. Additionally, exploratory analyses linked PFS with immune genes (CXCL9, TAP1) and PTEN.
Thus, are there some patients that may benefit from pembrolizumab + enzalutamide? In the KEYNOTE-641 trial, the dual primary endpoint of OS (median 24.7 months vs 27.3 months; HR 1.04, 95% CI 0.88−1.22) was not met, with no appreciable benefit noted in the subgroup analyses:
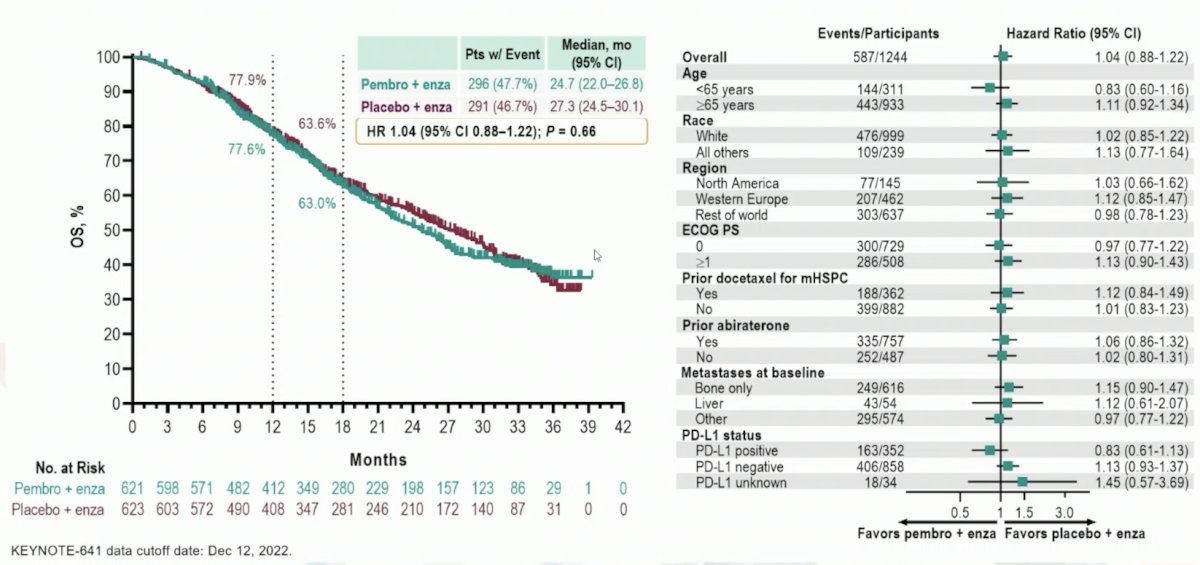
However, Dr. Beltran notes that numerically, there were more complete responses in the pembrolizumab + enzalutamide arm (7.4%) versus the placebo + enzalutamide arm (2.7%). Thus, the challenge is identifying these patients that may derive benefit. Of note, there are long term responders to IO single agent (ipilimumab) in this disease space.2 During long term follow-up, there was crossing of the curves at 7-8 months, followed by persistent separation of the curves favoring ipilimumab:
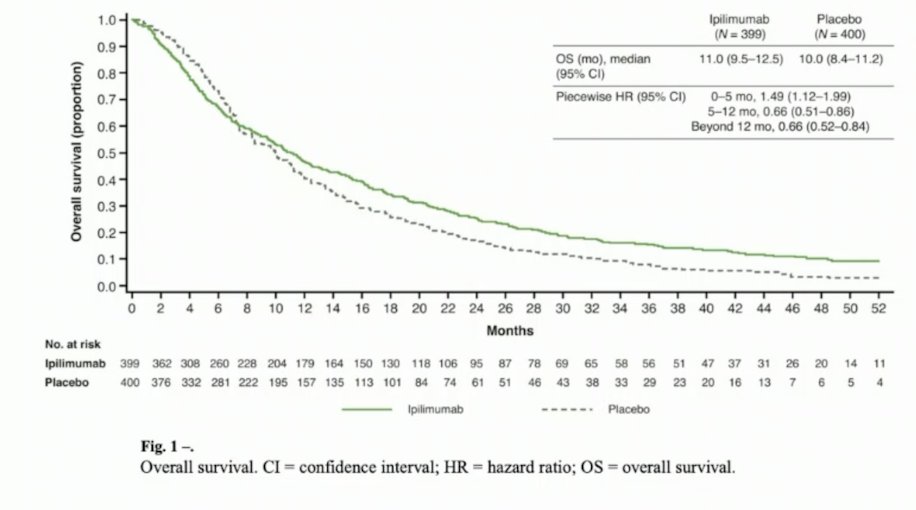
Indeed, the hazard ratio changed over time, initially 1.49 (95% CI 1.12-1.99) for 0-5 months, and then 0.66 (95% CI 0.51-0.86) for 5-12 months, and 0.66 (95% CI 0.52-0.84) beyond 12 months. Additionally, OS rates were higher with ipilimumab versus placebo at 2 years (25.2% vs 16.6%), at 3 years (15.3% vs 7.9%), at 4 years (10.1% vs 3.3%), and at 5 years (7.9% vs 2.7%). OS rates at 3, 4, and 5 years were 2-3 times higher in the ipilimumab arm.
With regards to biomarkers, TMB-high, MSI, dMMR are associated with exceptional response to IO, leading to pembrolizumab having pan cancer approval for these alterations. Additionally, high PD-L1 IC2/3 and CD8 expression were associated with response to atezolizumab + enzalutamide in the IMbassador 250 trial. In a phase 2 trial of enzalutamide followed by enzalutamide + pembrolizumab, scRNA-seq of metastatic lesions from eight men (3 responders and 5 non-responders) after enzalutamide prior to pembrolizumab showed no difference in CD4 T-cells, CD8 T-cells, NK, B cells or myeloid cell proportions, or tumor mutational burden to explain these differences. We know that T cells have an intrinsic role in AR regulation of IFN-gamma activity, which limits anti-tumor immunity and T-cell re-invigoration. Additionally, there is direct binding of AR to critical inflammatory gene enhancer regions in T cells. So, Dr. Beltran suggests that potentially there is an enhanced response to checkpoint inhibition with ADT in mHSPC.
Are there differences between CRPC and HSPC? Dr. Beltran then discussed the KEYNOTE-991 trial that randomized mHSPC men 1:1 to receive pembrolizumab 200 mg or placebo IV every 3 weeks for ≤35 cycles + enzalutamide 160 mg orally daily + continuous ADT (if no history of bilateral orchiectomy). The trial design for KEYNOTE-991 is as follows:

The primary endpoint of rPFS for pembrolizumab + enzalutamide vs placebo + enzalutamide was not met (median NR vs NR, HR 1.20, 95% CI 0.96−1.49):

Dr. Beltran offered the following possibilities for why this trial was negative:
- The acute immune infiltration after ADT may be transient
- Immunosuppressive cells predominate
- There are other immune checkpoints (ie. VISTA, B7-H3) that may be at play
- Only a small subset of patients may benefit
- How can we develop novel immunotherapy combinations?
The following are potential strategies to sensitize to immune checkpoint inhibition:

The phase 3 CONTACT-02 trial is randomizing men with mCRPC 1:1 to cabozantinib + atezolizumab versus abiraterone or enzalutamide with the following trial design:

Of note, on August 21, 2023, there was a press release for this trial stating “cabozantinib + atezolizumab demonstrated a statistically significant reduction in the risk of disease progression or death compared with a second novel hormonal therapy in patients with mCRPC. A trend toward improvement in OS was observed at the first interim analysis.” So, does Dr. Beltran feel that immunotherapy is over for prostate cancer? In her opinion, the answer is no, but what is needed is better biomarkers, continued investigation of combination therapies, and novel immune targets.
Dr. Beltran then discussed the final analysis of the MAGNITUDE trial. In the niraparib + abiraterone acetate + prednisone and placebo + abiraterone acetate + prednisone arms, 70% and 86% of patients received subsequent life-prolonging therapy. OS favored niraparib + abiraterone acetate + prednisone over placebo + abiraterone acetate + prednisone (HR 0.788, 95% CI 0.554-1.120):
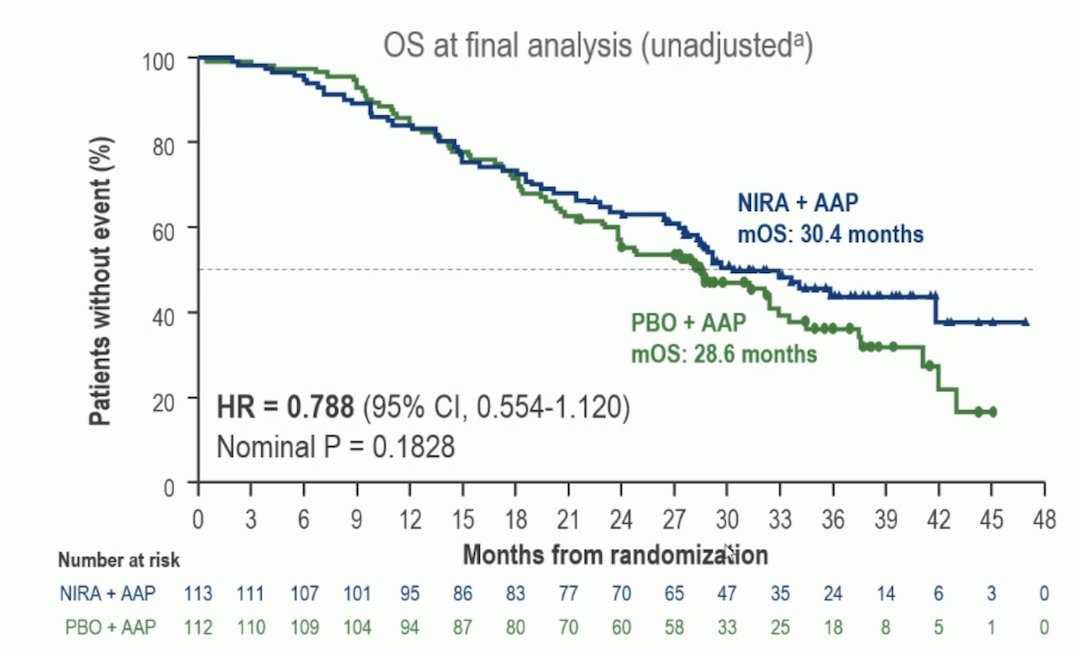
A prespecified multivariable analysis adjusting for baseline imbalances showed an OS benefit favoring niraparib + abiraterone acetate + prednisone (HR 0.663, 95% CI 0.464-0.947). Dr. Beltran’s take home points from the MAGNITUDE trial are as follows:
- Niraparib with abiraterone represents another new option for patients with BRCA alterations and was FDA approved on August 11, 2023
- It is very important to see this OS data
- Of note, 33.4% of patients in the control arm received PARP inhibitors and 9.3% received platinum-based chemotherapy
- This suggests that early combination therapy may be better than sequential treatment
Currently, the landscape for PARP inhibition in mCRPC is as follows:
First line CRPC:
- PROpel:3 olaparib + abiraterone (approved for all comers in Europe, approved for BRCA 1/2 in the US)
- TALAPRO-2:4 talazoparib + enzalutamide (approved for HRR positive mutations in the US)
- MAGNITUDE:5 niraparib + abiraterone (approved for BRCA 1/2 in the US)
Post-ARPI
- PROFOUND:6 olaparib (approved for a panel of HRR positive mutation genes in the US and BRCA in Europe)
- TRITON 2 and 3:7,8 rucaparib (approved for BRCA 1/2)
There are several open questions when thinking about PARP inhibitors and ARPI combinations:
- Are all PARP inhibitor combinations the same?
- Biomarker differences: BRCA vs non-BRCA vs non-HRR?
- What is the best way to test?
- What is the mechanism? Is there really AR-DNA repair cross talk translating to clinical benefit in select patients? Is this driven by single agent activity?
The following are arguments for and against cross talk:

With regards to adverse events in the MAGNITUDE trial, there were no cases of acute myeloid leukemia in the niraparib + abiraterone arm, and differences in safety between arms were driven by known hematologic toxicities of niraparib. Interestingly, pulmonary embolisms occurred in 4.7% of patients in the niraparib + abiraterone arm and in 1.4% in the placebo + abiraterone arm. Compared to other trials, there were 7% of patients in the PROpel trial receiving olaparib + abiraterone had a pulmonary embolism, 4% in the TALAPRO-2 trial receiving talazoparib + enzalutamide, and 4% of patients in the PROFOUND trial receiving olaparib.
Dr. Beltran provided the following applications of MAGNITUDE to current practice:
- Niraparib + abiraterone is a new standard of care for BRCA mutated mCRPC and adds to the growing armamentarium of options
- It supports DNA sequencing at first line mCRPC (if not earlier)
- If BRCA mutation is positive, data supports early co-targeting with ARPI
- If the patient has received prior ADT + abiraterone for mHSPC, should we add niraparib? Switch to enzalutamide + talazoparib? Switch to single agent PARP inhibition?
Dr. Beltran then discussed the exciting data from the ProBio randomized, biomarker-driven trial of androgen receptor pathway inhibitors or taxanes for patients mCRPC. In this trial, men with mCRPC were randomized based on genomic alterations in circulating tumor DNA in five biomarker signatures:
- AR wild type and TP53 wild type
- TP53 mutant
- DRD
- TMPRSS2:ERG fusion
- All biomarkers signatures combined

Androgen receptor pathway inhibitors (abiraterone and enzalutamide) and taxanes (docetaxel and cabazitaxel) were evaluated, using progression-free survival, by no longer clinically benefiting per PCWG3 criteria (PFS), as primary endpoint. Enrollment in the experimental group was stopped when the Bayesian probability of superiority reached prespecified thresholds. There were 193 men randomized: 64 to physician’s choice, 31 to AR pathway inhibitors, and 56 to taxanes. Subsequently, 77 patients were re-randomized: 28 to physician’s choice, 20 to AR pathway inhibitors, and 19 to taxanes.
The median estimated PFS was 11.1 months (90% Bayesian CI, 9.6 to 13.3) for androgen receptor pathway inhibitors compared with 7.4 months (90% CI, 6.7 to 8.4) in the physician’s choice arm and 6.9 months (90% CI 6.2 to 8.0) in the taxanes arm. The median estimated OS was 38.7 months (90% CI 31.1 to 54.1) for androgen receptor pathway inhibitors compared with 21.8 months (90% CI 19.2 to 26.3) in the physician’s choice arm, and 21.7 months (90% CI 19.0 to 26.1) in the taxanes arm:

With regards to progression free survival and biomarker signatures:
- AR wild type and TP53 wild type: Median estimated PFS was 16.1 months (90% CI 12.8 to 22.2) for androgen receptor pathway inhibitors compared with 9.2 months (90% CI 7.9 to 11.4) in the physician’s choice arm and 9.3 months (90% CI 7.6 to 12.5) in the taxanes arm
- TMPRSS2-ERG positive: Median estimated PFS was 12.8 months (90% CI 9.9 to 18.1) for androgen receptor pathway inhibitors compared with 7.2 months (90% CI 5.9 to 9.3) in the physician’s choice arm and 6.5 months (90% CI 5.2 to 8.5) in the taxanes arm
- TP53-altered: Median estimated PFS was 6.8 months (90% CI 5.8 to 8.4) for androgen receptor pathway inhibitors compared with 6.0 months (90% CI 5.2 to 7.1) in the physician’s choice arm and 6.4 months (90% CI 5.6 to 7.7) in the taxanes arm
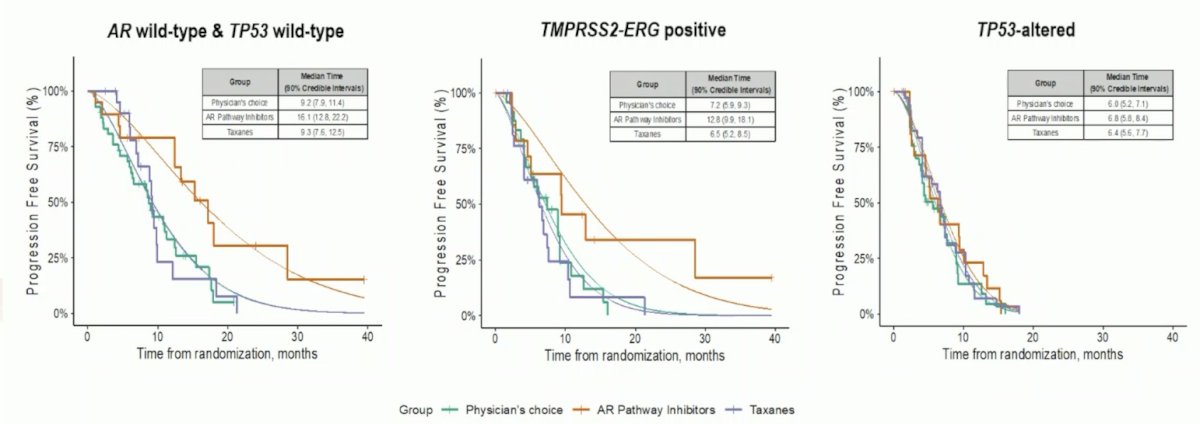
Dr. Beltran notes that the strength of this trial is (i) the innovative clinical trial design since we need more biomarker driven trials, (ii) the adaptive approach which can expand disease states and biomarkers, and (iii) demonstrating the feasibility of such a complex study across multiple institutions. There are, however, several open ended questions:
- There are still relatively few numbers in each arm and multiple comparisons – what is driving these differences?
- Targets are standard (ARPI, taxane), but may establish a framework for new drugs
- ctDNA results were not used for treatment selection, so how do we translate into predictive biomarkers?
There are several comparable trials in this disease space, including PC-BETS, PREDICT, and GUNS. Dr. Beltran concluded here discussant presentation by highlighting that the best theme for tying these abstracts together is: Patient Selection is Critical
Presented by: Himisha Beltran, MD, Dana-Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Powles T, Yuen KC, Gillessen S, et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: A randomized phase 3 trial. Nat Med. 2022 Jan;28(1):144-153.
- Fizazi K, Drake CG, Beer TM, et al. Final analyses of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur Urol. 2020 Dec;78(6):822-830.
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomized, double-blind, phase 3 trial. Lancet Oncol. 2023 Oct;24(10):1094-1108.
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomized, placebo-controlled, phase 3 trial. Lancet. 2023 Jul 22;402(10398):291-303.
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023 Jun 20;41(18):3339-3351.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020 May 28;382(22):2091-2102.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol 2020 Nov 10;38(32):3763-3772.
- Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N Engl J Med. 2023 Feb 16 [Epub ahead of print].
ESMO 2023: KEYNOTE-641 Phase 3: Pembrolizumab plus Enzalutamide for Patients with Metastatic Castration-Resistant Prostate Cancer
ESMO 2023: KEYNOTE-991 Pembrolizumab plus Enzalutamide and Androgen Deprivation Therapy or Patients with Metastatic Hormone-Sensitive Prostate Cancer
ESMO 2023: MAGNITUDE Three-Year Update and Final Analysis: Niraparib with Abiraterone Acetate plus Prednisone as First-Line Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer and Homologous Recombination Repair Gene Alteration
ESMO 2023: ProBio, a Randomized, Outcome-Adaptive, Biomarker-Driven Platform Trial: AR Pathway Inhibitors or Taxanes for Patients with Metastatic Castration-Resistant Prostate Cancer: A Direct Comparison


