(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a proffered paper session for non-prostate genitourinary malignancies. Dr. Hiroshi Kitamura presented the results of JCOG1019, an open-label, non-inferiority, randomized phase III trial comparing the effectiveness of watchful waiting and intravesical Bacillus Calmette-Guérin (BCG) in patients with high-grade T1 bladder cancer with evidence of pT0 disease on the repeat transurethral resection specimen.
T1 bladder cancer, characterized by invasion through the subepithelial layer, accounts for approximately 20% of non-muscle invasive bladder cancer (NMIBC) cases and presents a particular clinical challenge owing to its aggressive biological behavior and elevated risk of recurrence and progression. Transurethral resection of bladder tumour (TURBT) followed by a second transurethral resection (TUR) and intravesical bacillus Calmette-Guérin (BCG) has been the standard treatment for T1 bladder cancer without very-high-risk features, irrespective of the pathological results on the second TUR.
To date, there is no evidence to support the routine use of BCG for patients with high-grade pT1 bladder cancer who have pT0 histology after the second TUR. Dr. Kitamura noted that intravesical BCG therapy has potential side effects and there have been wide world availability/supply issues. There is an ongoing shortage of BCG, necessitating strategies to prioritize its use in patients with NMIBC.
JCOG1019 is a phase III trial of patients with BCG-naïve, high-grade T1 urothelial carcinoma of the bladder following a complete TURBT. Eligible patients were to undergo a second TUR. If they had evidence of pT0 on the repeat TUR, then they were randomized 1:1 to watchful waiting versus intravesical BCG weekly x 8 doses (80 mg, Tokyo-172 strain or 81 mg, Connaught strain). The primary endpoint was relapse-free survival, excluding Tis or Ta intravesical recurrence. With regards to the statistical plan for analysis, non-inferiority would be demonstrated if the upper limit of the two-sided 90% confidence interval (CI) of the hazard ratio (HR) was less than 1.60. The planned sample size for randomization was 260, with a one-sided alpha of 0.05, power of 70%, and non-inferiority margin of 1.60 in terms of HR.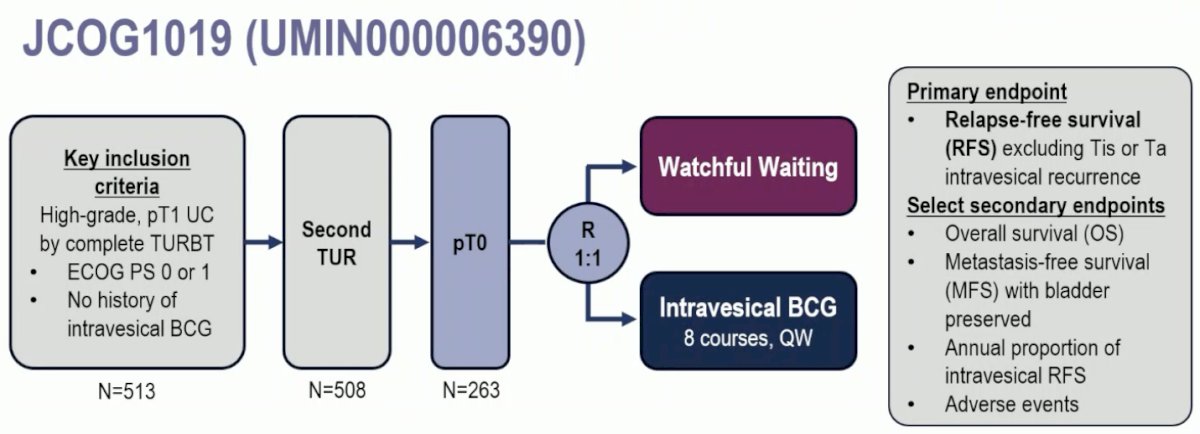
Of 513 eligible patients, 508 underwent a second TUR. Of these 508 patients, 263 had evidence of T0 disease on the repeat resection. The baseline patient characteristics are summarized below. 96% of patients had primary bladder cancer. ~50% had multifocal disease.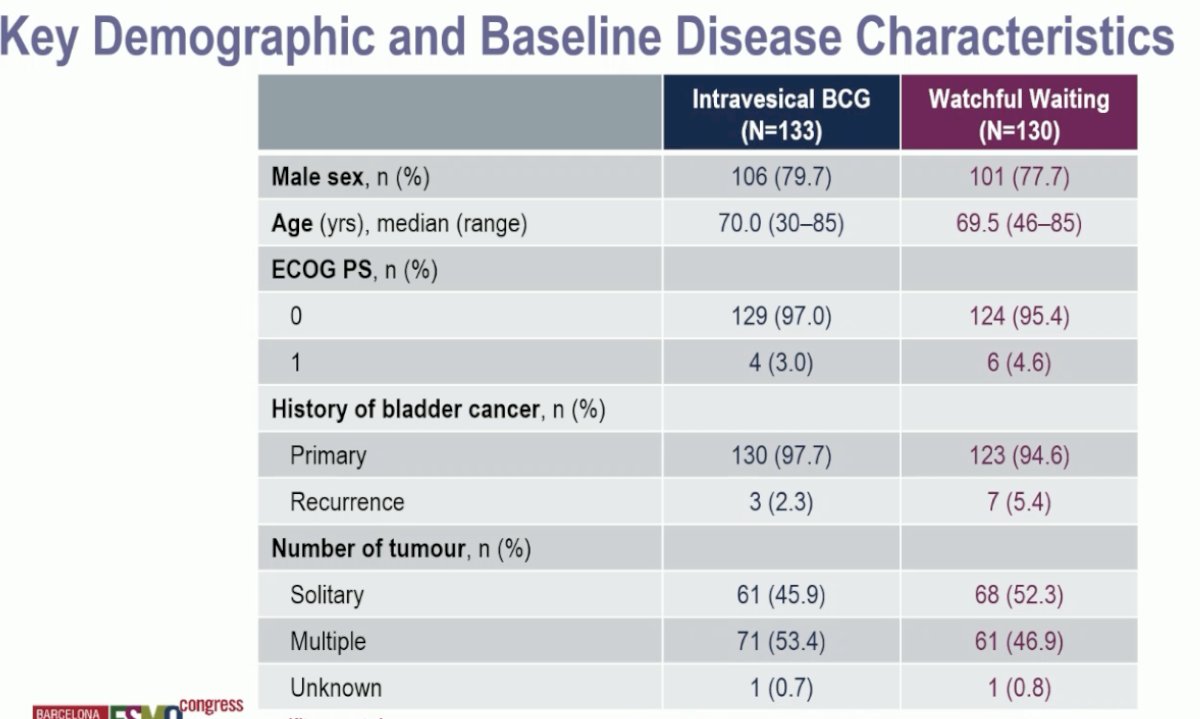
Of the 133 patients in the intravesical BCG arm, 93% of patients received any amount of study drug. 82% completed all 8 cycles of BCG. The primary reason for BCG discontinuation was the occurrence of an adverse event (16.5%).
Watchful waiting was non-inferior to intravesical BCG for recurrence-free survival (excluding Tis or Ta intravesical recurrence), with an HR of 0.69 (90% CI: 0.44–1.08; one-sided p-value for non-inferiority=0.00102). The 5-year recurrence-free survival rates were 87% and 82% for watchful waiting and intravesical BCG, respectively.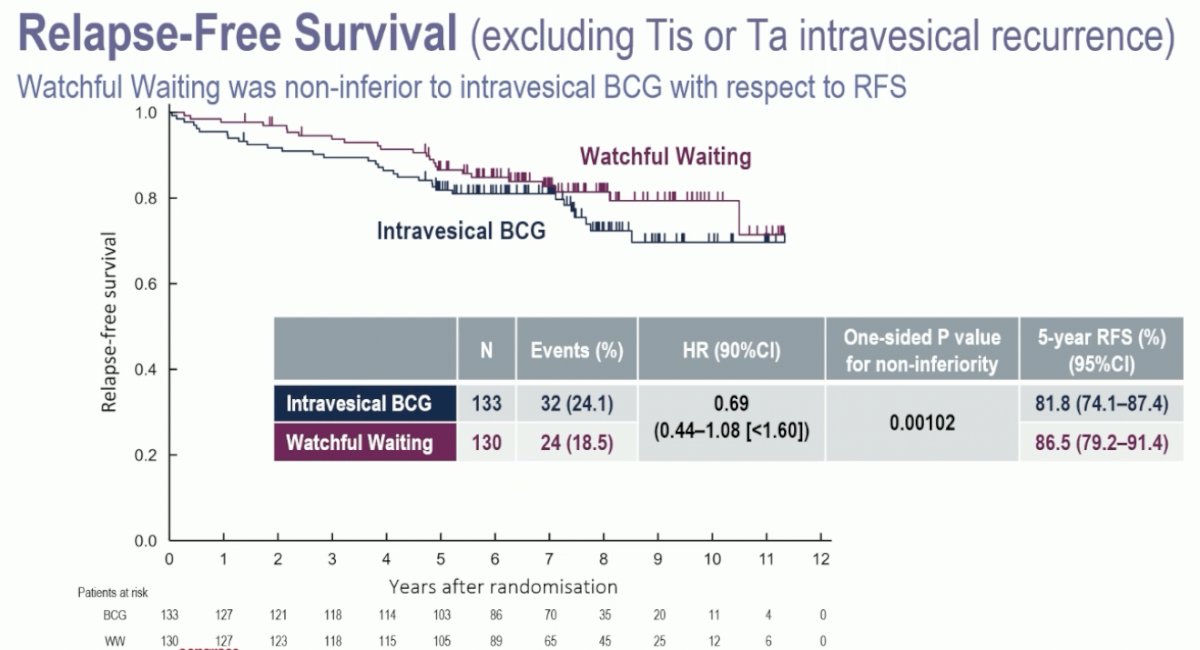
Subgroup analyses demonstrated a consistent non-inferiority for watchful waiting. The only possible exception to this was younger patients (age <65 years), who had a recurrence-free survival HR of 2.82 (95% CI: 0.87–9.15) with watchful waiting, compared to BCG as reference.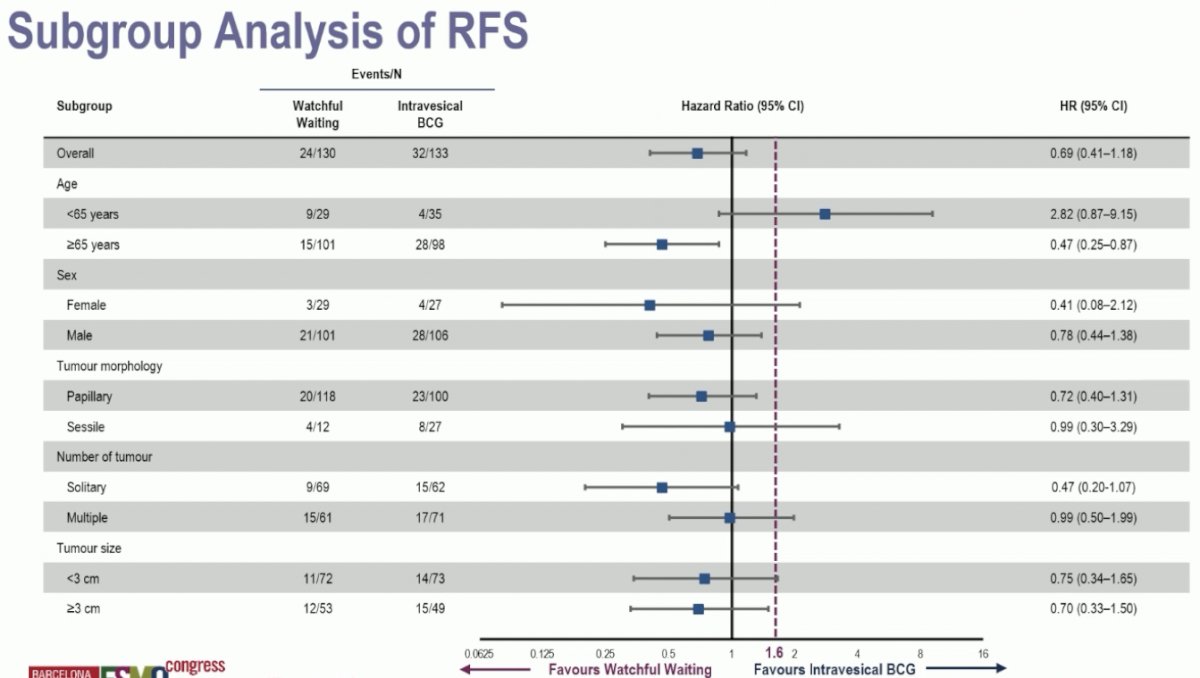
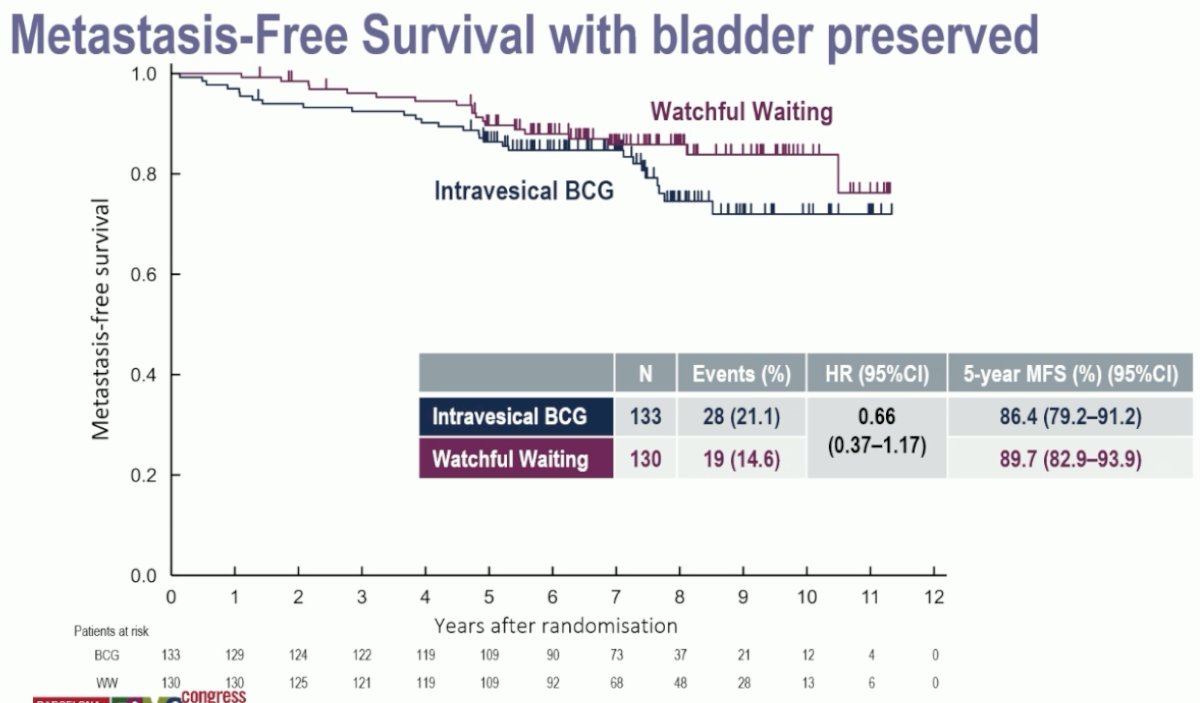
Similarly, there were no differences in overall survival or metastasis-free survival between the two arms:
For any intravesical relapse-free survival, there was a lower rate of any relapse with intravesical BCG (HR: 1.33, 95% CI: 0.90–1.97), with 55 relapses in the watchful waiting arm and 46 in the intravesical BCG arm.
With regards to adverse events, there were no new safety signals.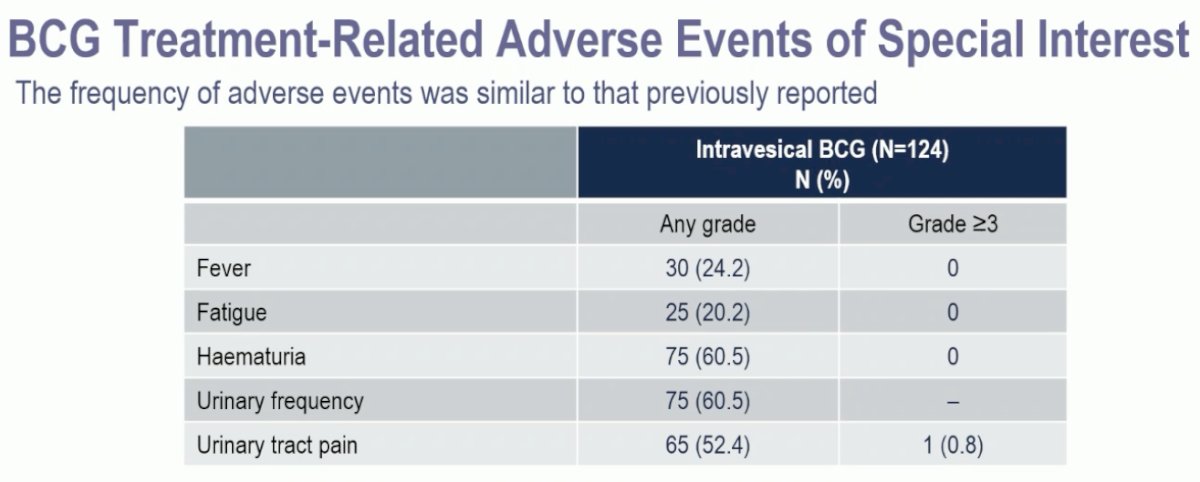
Grade 3 anemia was observed in two patients (1.7%), grade 3 urinary tract pain in one patient (0.8%), grade 3 renal infection in two patients (1.7%), grade 3 cytokine release syndrome in one patient (0.8%), grade 3 vesicular dermatitis in one patient (0.8%) and grade 3 arthritis in one patient (0.8%). No deaths occurred during the protocol treatment or within 30 days of the last treatment.
Grade ≥3 adverse events occurring after 31 days following the end of protocol treatment are summarized below:
Dr. Kitamura concluded as follows:
- Watchful waiting demonstrated statistically significant non-inferiority to intravesical BCG in for recurrence-free survival, excluding Tis or Ta recurrence, in patients with bladder cancer with high-grade T1 at the initial TURBT and pT0 at the second TUR
- Overall survival and metastasis-free survival were similar with watchful waiting or BCG
- Intravesical BCG tended to show better intravesical recurrence-free survival, compared to watchful waiting
- The safety profile of watchful waiting was better than that of intravesical BCG
- These results support watchful waiting as a potential new standard of care for patients with high-grade T1 bladder cancer without residual tumor at the second TUR.
Presented by: Hiroshi Kitamura, MD, Professor and Chairman, Department of Urology, Graduate School of Medicine and Pharmaceutical Sciences for Research, University of Toyama, Japan
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
Related Content:
JCOG1019 Trial Examines Watchful Waiting vs BCG for High-Grade T1 Bladder Cancer - Hiroshi Kitamura
ESMO 2024: Invited Discussant: JCOG1019, TOMBOLA, and SunRISe-4


