(UroToday.com) The 2024 European Society for Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain between September 13th and 16th, 2024 was host to a proffered paper session for non-prostate genitourinary malignancies. Dr. Bogdana Schmidt delivered the discussant session for JCOG1019, TOMBALA, and SunRISe-4.
Dr. Schmidt began by noting that high-grade pT1 progresses to muscle-invasive disease in 21% to 53% of patients. The bladder cancer-specific mortality for this subgroup ranges between 14% and 34%. The use of adjuvant intravesical BCG reduces the risk of recurrence from 51% to 26% and the risk of progression from 13.8% to 8.8% (relative risk reduction: 27%).1-3
JCOG1019 is a phase III trial of patients with BCG-naïve, high-grade T1 urothelial carcinoma of the bladder following a complete TURBT. Eligible patients were to undergo a second TUR. If they had evidence of pT0 on the repeat TUR, then they were randomized 1:1 to watchful waiting versus intravesical BCG weekly x 8 doses (80 mg, Tokyo-172 strain or 81 mg, Connaught strain). Dr. Schmidt highlighted that this BCG regimen is distinct from that currently recommended by numerous guidelines, including the EAU and AUA, which recommend an induction course of 6 weeks, followed by 3 years of maintenance for these high-risk patients. The primary endpoint was relapse-free survival, excluding Tis or Ta intravesical recurrence.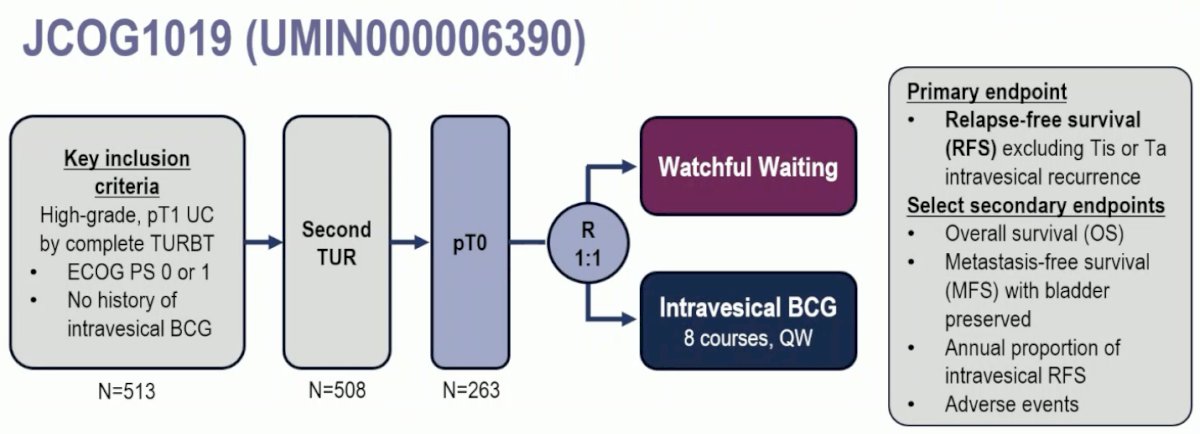
Approximately 50% of patients had cT0 on the repeat TUR specimen. Watchful waiting was non-inferior to intravesical BCG for recurrence-free survival (excluding Tis or Ta intravesical recurrence), with an HR of 0.69 (90% CI: 0.44–1.08; one-sided p-value for non-inferiority=0.00102). The 5-year recurrence-free survival rates were 87% and 82% for watchful waiting and intravesical BCG, respectively. Similarly, there were no differences in overall survival or metastasis-free survival between the two arms.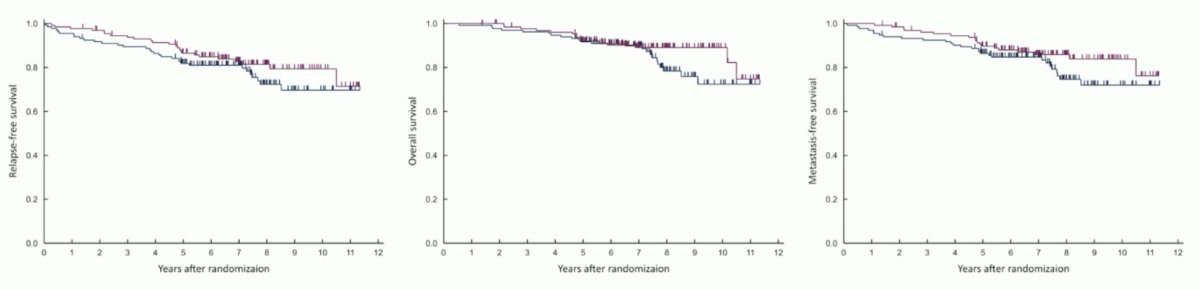
However, Dr. Schmidt noted that ~18% of patients in the BCG arm and ~14% in the watchful waiting arm recurred with T1 disease or progressed to MIBC, which is slightly higher than expected with adjuvant BCG.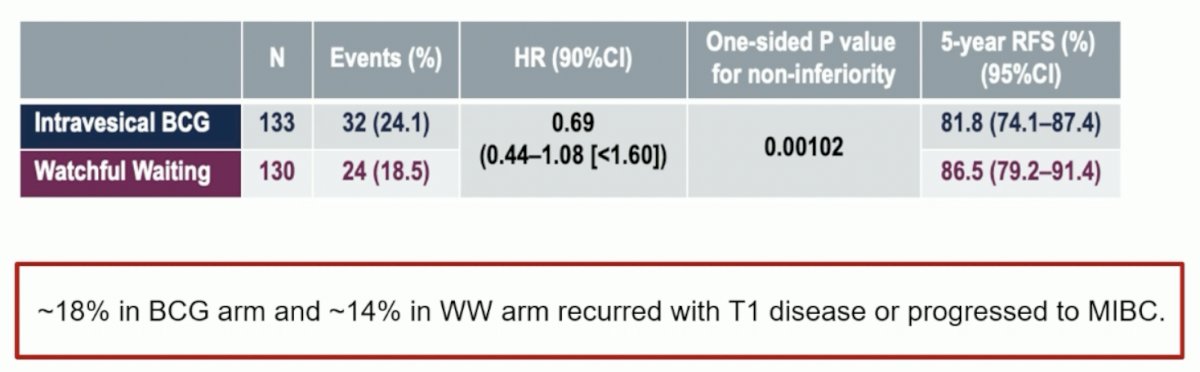
While there was no difference in metastasis-free survival between the two arms, the number of metastatic events in each arm was higher than would be expected for this population (15% and 21%).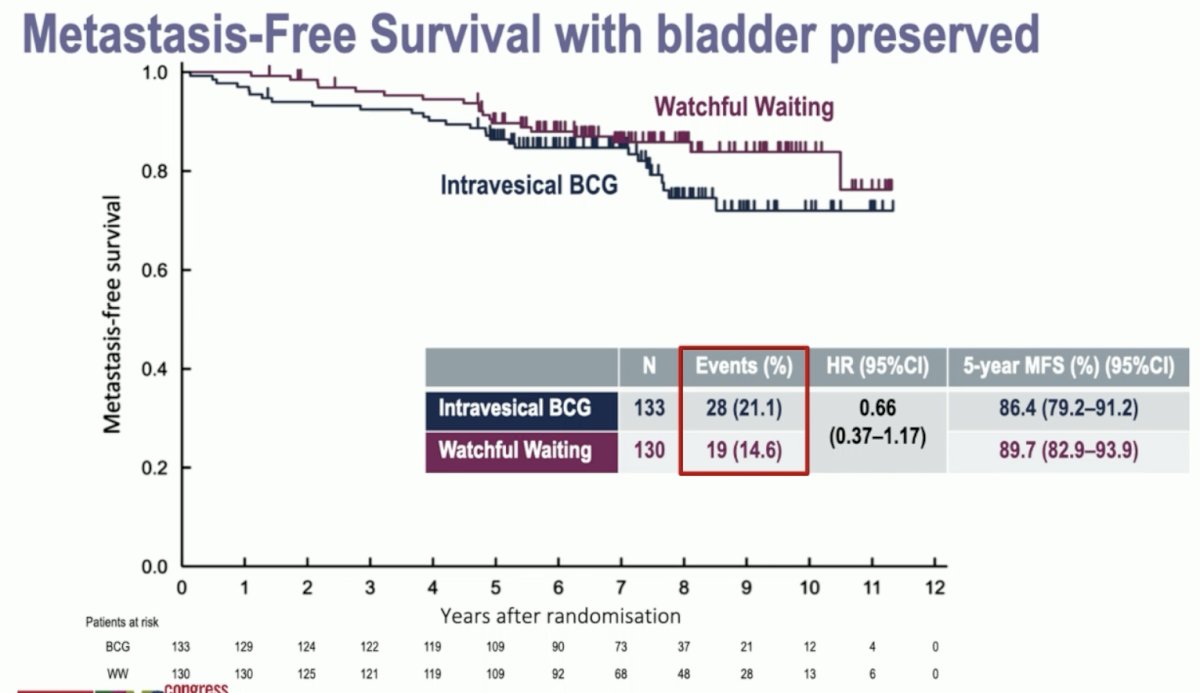
Are the results of JCOG1019 practice changing? While the recurrence-free (Ta/CIS excluded), metastasis-free, and overall survivals look similar between the groups, she argued that this study evaluates ‘no treatment’ versus ‘undertreatment’. The number of events (metastasis) is higher than that reported in other published studies. There is no bladder cancer-specific survival data presented. Furthermore, we need to wait for the final analysis to evaluate loss to follow-up patterns. Dr. Schmidt acknowledged that there is a subset of patients whose disease seems cured with high-quality TUR without adjuvant treatment, but we need better markers to identify these patients to manage them with watchful waiting.
Next moving on to discuss the TOMBOLA trial, Dr. Schmidt noted that we now have three phase III trials that have evaluated adjuvant immunotherapy following radical resection in patients with muscle-invasive urothelial carcinoma at high risk of recurrence. Two of these three trials (AMBASSADOR and CheckMate 274) were positive for a disease-free survival benefit.4,5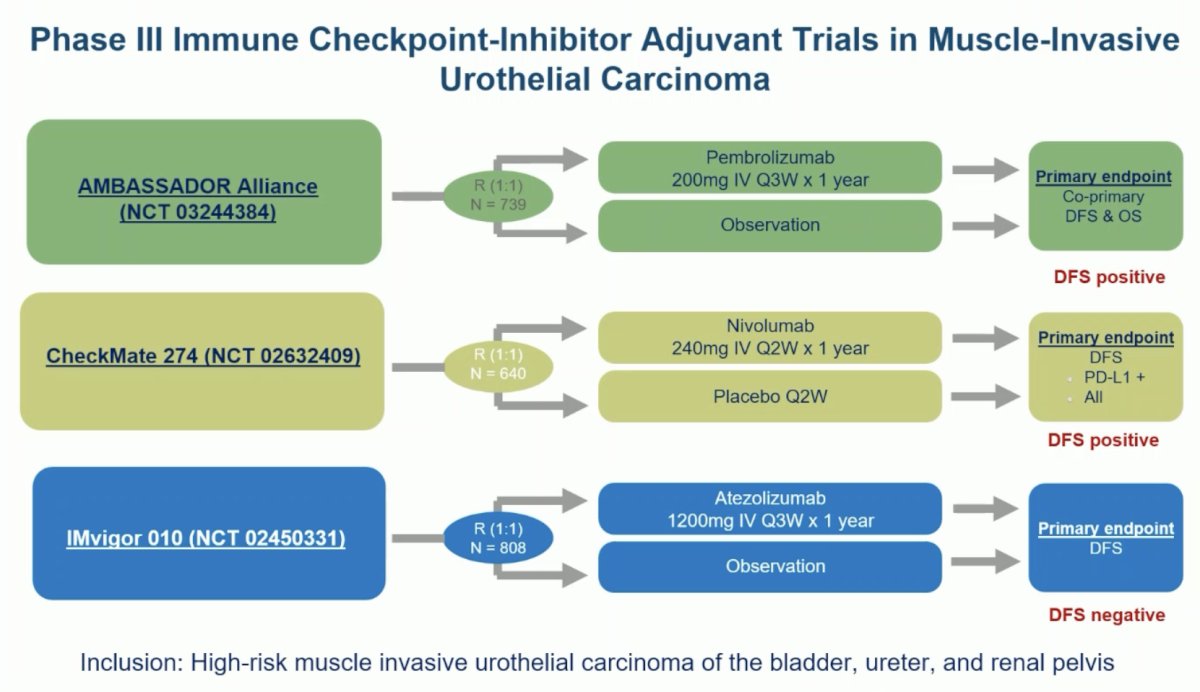
Important lessons from these trials are that not all patients require adjuvant immunotherapy and approximately one-third of patients remained disease-free at 3 years in the placebo/surveillance arms, highlighting the need for de-escalation strategies.
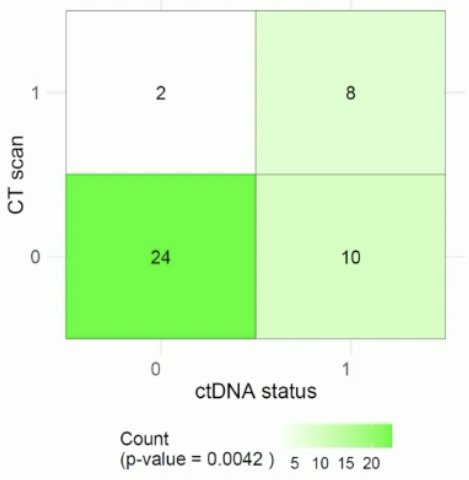
TOMBOLA was a national, non-randomized ctDNA-based intervention study conducted at 5 centers in Denmark. Eligible patients were those with cT2-4aN0-1M0, cisplatin, and immunotherapy-eligible MIBC who underwent NAC followed by radical cystectomy. Patients underwent serial ctDNA testing post-operatively. Upon ctDNA detection, patients were recommended for one year of atezolizumab therapy. The primary objective was a complete response after treatment with the investigational agent initiated by ctDNA positive status after radical cystectomy. A complete response was defined by both a negative ctDNA status and no visible metastasis on CT.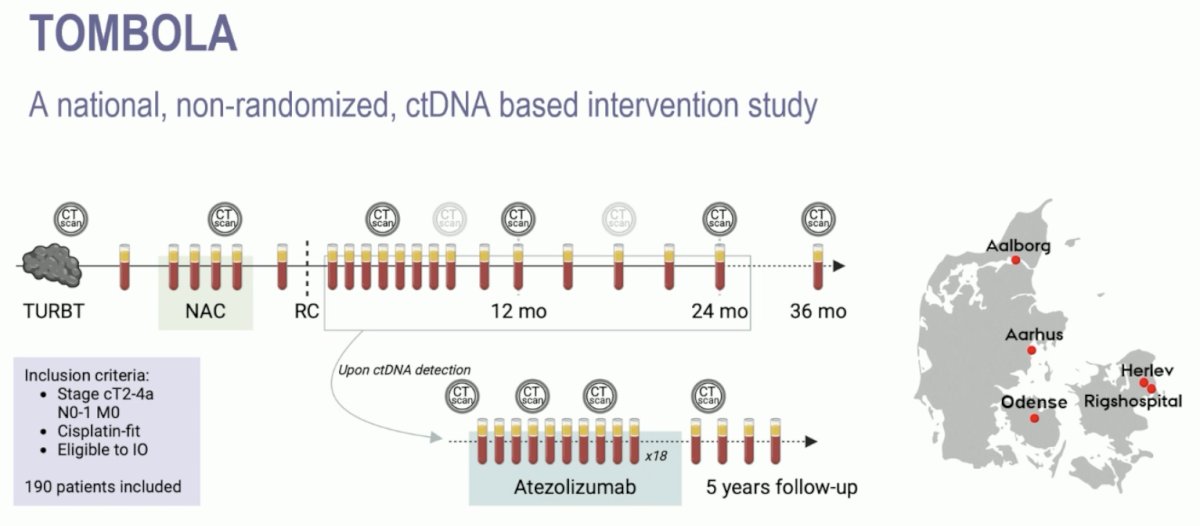
Notable findings from this study included:
- 52% were ctDNA+ post-radical cystectomy
- 75% were detected < 4 months post-radical cystectomy
- Of the ctDNA- patients, only 2 (3%) developed metastases (different from the IMvigor011 trial that demonstrated a 10% metastatic rate in ctDNA- patients)6
- 55% of patients met the primary endpoint of no evidence of disease
She noted that the recurrence-free survival outcomes were excellent in the ctDNA- patients, illustrated below. How do these results compare to those from IMvigor010, stratified by ctDNA status? Overall the results from TOMBOLA are better, which might be an early signal that this ctDNA biomarker-directed approach may have real survival benefits.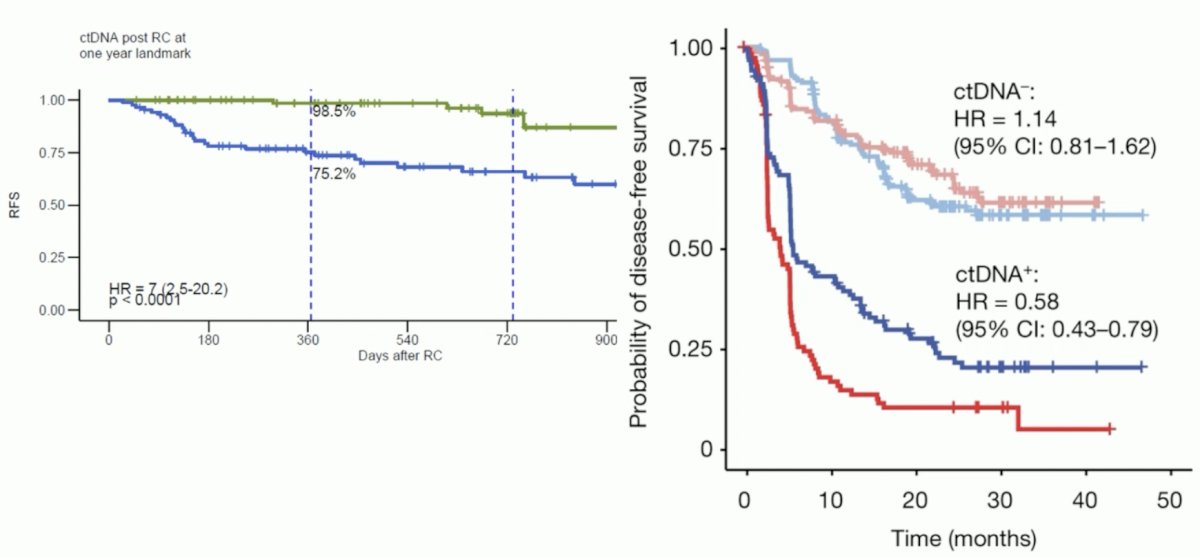
Are the results of TOMBOLA practice changing? While we need a way to stratify patients by who will most benefit from adjuvant treatment and these findings suggest that serial ctDNA could help stratify these patients, monthly serial ctDNA does not seem clinically practical, both timewise and financially. There must be predictors of treatment response or rapid ctDNA clearance, as seen in the IMvigor010 exploratory analysis that we need to identify. This is now data confirming that we can use ctDNA to impact management decisions, but does it change the outcome?
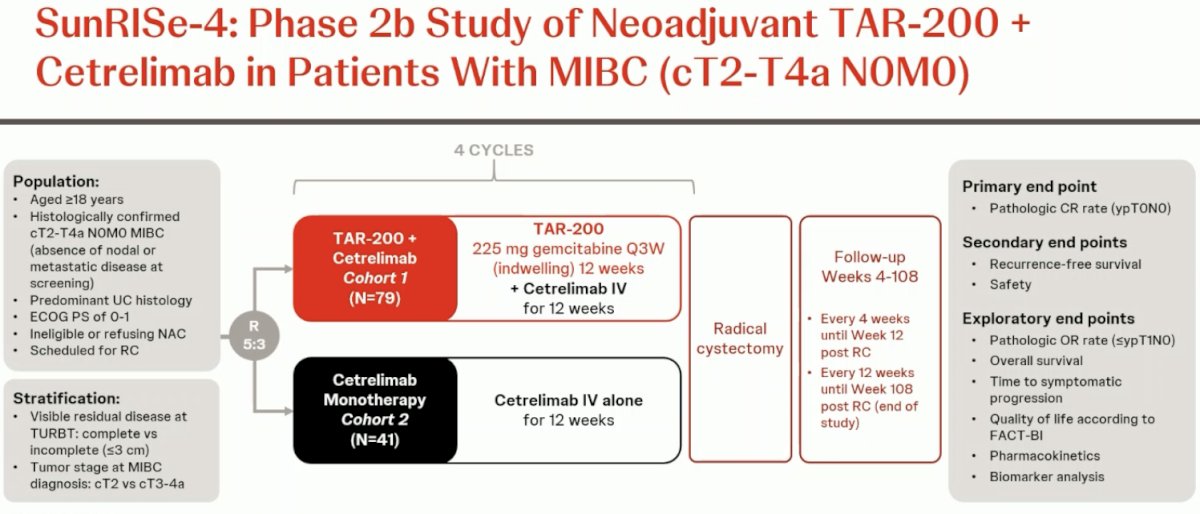
Next, moving on to discuss SunRISe-4 (NCT04919512), this is an ongoing randomized phase II study assessing the efficacy and safety of neoadjuvant TAR-200 + cetrelimab or cetrelimab monotherapy in patients with MIBC scheduled for radical cystectomy and who are ineligible for or refuse NAC.
The study design is summarized below. The key trial eligibility criteria were as follows:
- cT2-4aN0M0 MIBC
- Predominant urothelial carcinoma histology
- Ineligible for or refusing NAC
ECOG performance status 0–1
Patients underwent 5:3 randomization to:
- TAR-200 225 mg gemcitabine every 3 weeks (indwelling) for 12 weeks + cetrelimab intravenously for 12 weeks (4 cycles)
- Cetrelimab intravenously for 12 weeks (4 cycles)
Randomization was stratified by the absence or presence of visible residual disease at TURBT and tumor stage at MIBC diagnosis (cT2 versus cT3-4a). The planned sample size was n=160. The primary endpoint was a pathologic complete response. Key secondary endpoints were recurrence-free survival, safety, pathologic objective response (pOR; i.e., ≤ypT1N0), and overall survival. For this interim analysis, the clinical data cutoff was May 31, 2024.
She noted that only five patients in the entire cohort progressed (1.3% in Cohort 1 of combination TAR-200 + cetrelimab and 9.5% in Cohort 2 of cetrelimab monotherapy). From a patient characteristics standpoint, ~60% of patients were NAC refusing and 20–27% had tumors with variant histology.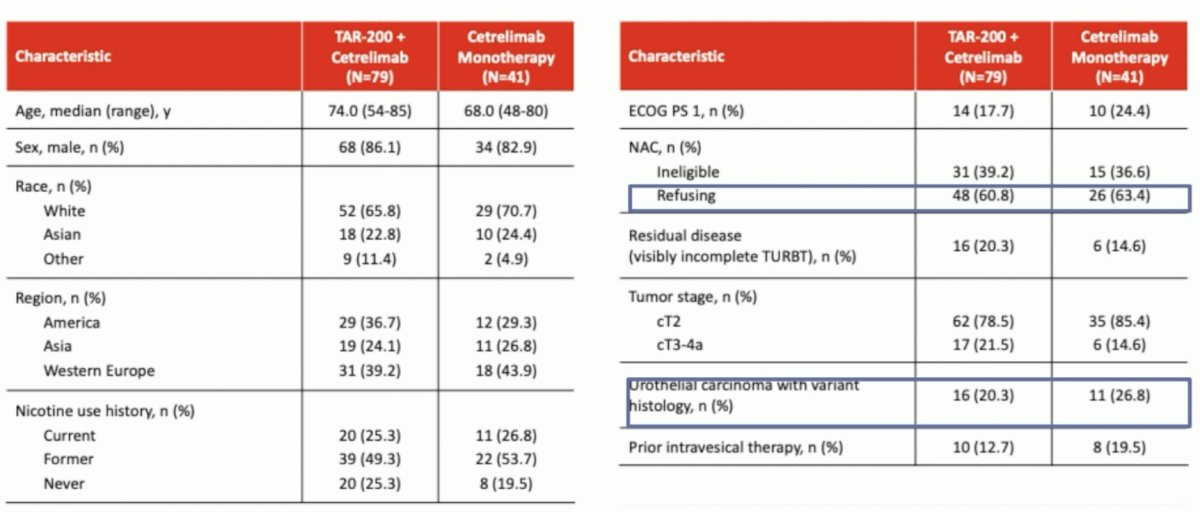
Notable results from this trial included:
- TAR 200 + cetrelimab:
- pCR 42% overall (23% in сТЗ/Т4, 48% in сТ2)
- pCR 56% for incomplete TUR versus 39% for complete
- Is this secondary to a priming effect or is it simply ‘noise’ from small numbers?
- Cetrelimab alone:
- pCR 23%
- PURE-01 with pembrolizumab demonstrated 42%, albeit slightly different populations and with higher treatment-related adverse events7
- pCR 23%
Framing the results of SunRISe-4 within the context of other neoadjuvant combination trials, we see that the combination of TAR-200 + cetrelimab had a more favorable safety profile with comparable efficacy outcomes.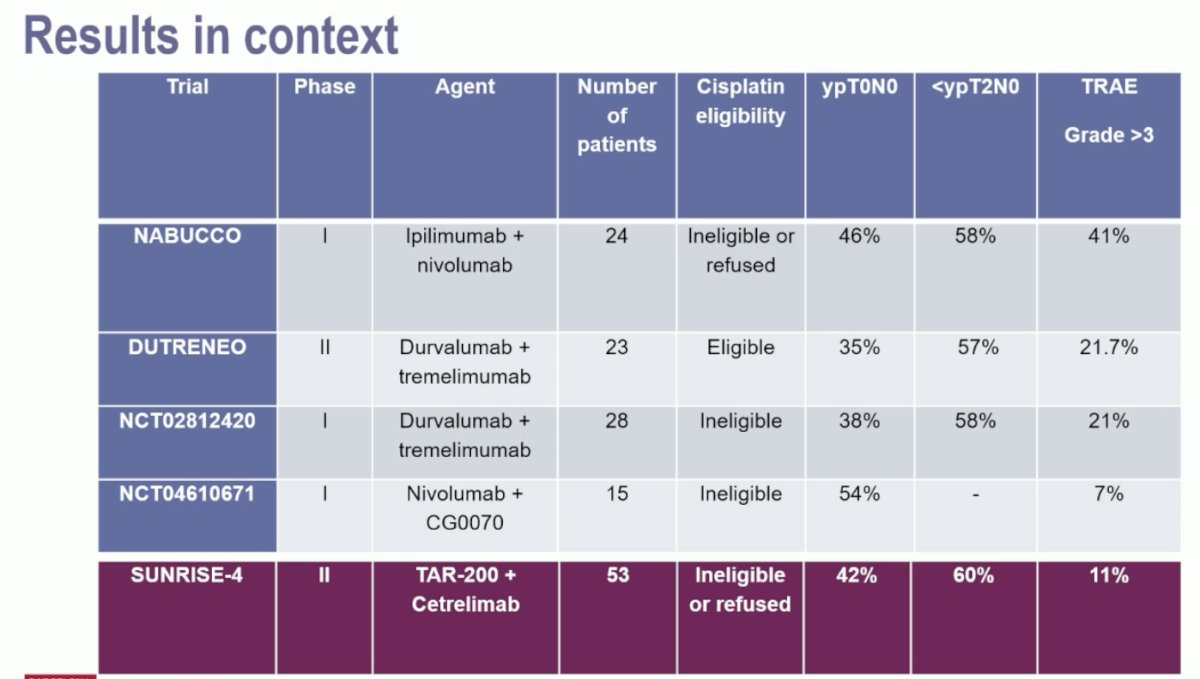
Are the results of SunRISe-4 practice changing? Dr. Schmidt argued that they potentially could be, although we need more information about the patients whose disease progressed or died prior to cystectomy. She also noted that with more experience and best supportive care, there was an improved dose delivery of TAR-200, which may translate to better outcomes. Further analyses of biomarkers associated with response are needed. Interestingly, as we refine and define our biomarkers, could we use these results in the future to select patients with durable benefits for consideration of bladder preservation?
Presented by: Bogdana Schmidt, MD, MPH, Assistant Professor of Urology, Department of Urology, University of Utah, Salt Lake City, UH
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:- Shelley MD, Court JB, Kynaston H, et al. Intravesical Bacillus Calmette-Guerin in Ta and T1 Bladder Cancer. Cochrane Database Syst Rev. 2000; 2000(4): CD001986.
- Klaassen Z, Kamat AM, Kassouf W, et al. Treatment Strategy for Newly Diagnosed T1 High-Grade Bladder Urothelial Carcinoma: New Insights and Updated Recommendations. Eur Urol. 2018; 74(5):597-608.
- Sylvester RJ, van der Meijden APM, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002; 168(5):1964-70.
- Apolo AB, Ballman KV, Sonpavde G, et al. Adjuvant Pembrolizumab versus Observation in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2024.
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021; 384(22):2102-2114.
- Powles T, Assaf ZJ, Degaonkar V, et al. Updated Overall Survival by Circulating Tumor DNA Status from the Phase 3 IMvigor010 Trial: Adjuvant Atezolizumab Versus Observation in Muscle-invasive Urothelial Carcinoma. Eur Urol. 2024; 85(2):114-22.
- Necchi A, Raggi D, Gallina A, et al. Updated Results of PURE-01 with Preliminary Activity of Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Carcinoma with Variant Histologies. Eur Urol. 2020; 77(4):439-46.
ESMO 2024: TAR-200 plus Cetrelimab or Cetrelimab Alone as Neoadjuvant Therapy in Patients with Muscle-Invasive Bladder Cancer Who Are Ineligible for or Refuse Neoadjuvant Cisplatin-Based Chemotherapy: Interim Analysis of SunRISe-4
ESMO 2024: Identification of Bladder Cancer Patients That Could Benefit from Early Post-Cystectomy Immunotherapy Based on Serial Circulating Tumour DNA Testing: Preliminary Results from the TOMBOLA Trial
ESMO 2024: JCOG1019 Phase III Study Comparing the Effectiveness of Watchful Waiting and Intravesical BCG in Patients with High-grade pT1 Bladder Cancer with pT0 on the 2nd Transurethral Resection Specimen
Related Content:
SunRISe-4 Trial Explores TAR-200 and Cetrelimab Combination for Bladder Cancer Treatment - Andrea Necchi


