(UroToday.com) The 2024 European Society of Medical Oncology (ESMO) Annual Congress held in Barcelona, Spain was host to the session Biochemical failure post-local therapy: An opportunity for tailored treatment? Dr. Daniela E. Oprea-Lager discussed if PSMA PET could be a game changer for treatment decision-making in patients with biochemical failure post-local therapy.
Dr. Oprea-Lager began her presentation by saying that prostate-specific membrane antigen (PSMA) positron emission tomography (PET) imaging is emerging as a game changer in the management of prostate cancer, revolutionizing how we approach this disease. PSMA PET offers a more personalized imaging approach, allowing for tailored treatment strategies based on individual patient profiles. This molecular imaging technique enhances patient management by providing precise localization of prostate cancer lesions, which improves decision-making and treatment planning. Additionally, PSMA PET also contributes to more accurate molecular imaging reporting and standardization. PSMA PET has been included in the most updated clinical practice guidelines related to prostate cancer.
The EAU-EANM-ESTRO-ESUR-SIOG guidelines for prostate cancer screening, diagnosis, and local treatment introduced PSMA PET/CT in 2020. The guidelines endorse using PSMA PET/CT when PSA levels exceed 0.2 ng/mL after radical prostatectomy, provided the results will impact subsequent treatment decisions. If PSMA PET/CT is not available and PSA levels are ≥1 ng/mL, fluciclovine PET/CT or choline PET/CT should be considered. For patients with recurrence following radiotherapy, the guidelines suggest utilizing PSMA PET/CT if accessible. However, it is important to note that the initial recommendations for PSA levels >0.2 ng/mL and ≥1 ng/mL are considered weak.
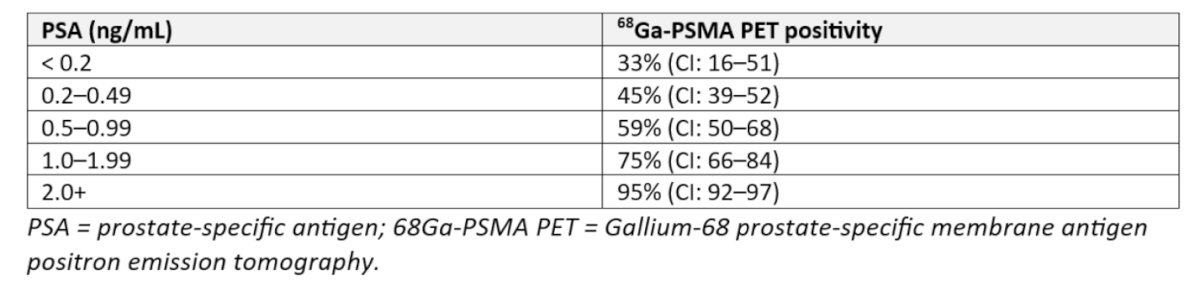
It is crucial to highlight that the positivity of PSMA PET scans is closely linked to the pre-PET PSA level, as consistently demonstrated in studies. For instance, patients with a PSA level <0.2 ng/mL have a 33% likelihood of a positive 68Ga PSMA PET scan, whereas 59% of patients with a PSA level between 0.5 and 0.99 ng/mL will have a positive scan after radical prostatectomy recurrence. A systematic review and meta-analysis by Marlon Perera and colleagues explored predictors for positive 68Ga-PSMA PET scans found that the likelihood of a positive scan increases with higher pre-PET PSA levels. Specifically, positivity rates were 42% for PSA levels 0–0.2 ng/mL, 58% for 0.2–1 ng/mL, 76% for 1–2 ng/mL, and 95% for PSA levels >2 ng/mL, we have to keep in mind that Choline PET could only achieved this positivity with PSA levels above 4 ng/ml. Additionally, a shorter PSA doubling time was associated with higher 68Ga-PSMA PET positivity, and both the sensitivity and specificity of PSMA PET were found to be 86%.(2)
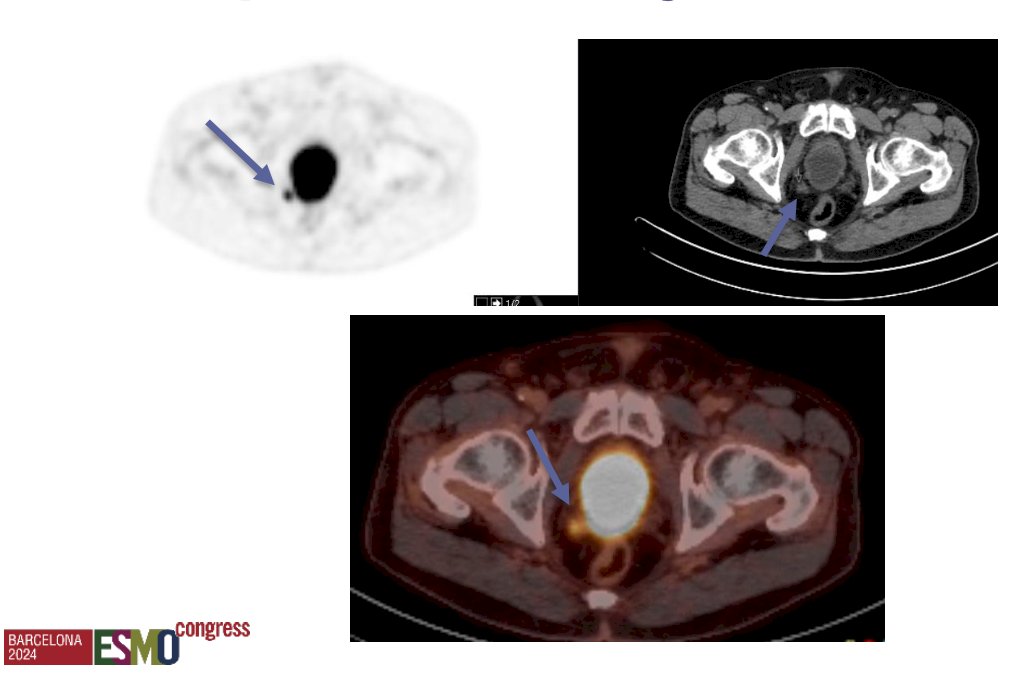
This raises the question of the optimal timing for performing a PSMA PET/CT. Dr. Oprea-Lager highlighted a case of a patient with biochemical recurrence post-radical prostatectomy who had a PSA level of 0.3 ng/mL. Despite this relatively low PSA level, a mesorectal lymph node was identified on the PSMA PET/CT.
The Osprey trial (NCT02981368) was a phase 2/3 prospective study evaluating PSMA PET/CT with piflufolastat F 18 (also known as 18F-DCFPyL or PyL). This trial included two cohorts: Cohort A comprised NCCN high-risk prostate cancer patients scheduled for radical prostatectomy with pelvic lymphadenectomy, while Cohort B consisted of men with radiologically suspected recurrent or metastatic prostate cancer. Participants received a single intravenous dose of 9 mCi (333 MBq) of piflufolastat F 18, followed by PET/CT acquisition 1-2 hours later. A total of 385 subjects were enrolled, including 117 men in Cohort B, 37% of whom were hormone-therapy naïve. Of these 117 patients, 93 were evaluable, demonstrating an overall sensitivity of 95.8% and a positive predictive value of 81.9%. These results were consistent across all sites of disease and PSA ranges. (3)

Dr. Oprea-Lager discussed the CONDOR trial (NCT03739684), a phase 3, open-label, single-arm, multicenter study evaluating the performance of 18F-DCFPyL PET/CT in 208 men with biochemically recurrent prostate cancer. The primary endpoint was the correct localization rate (CLR), defined as the positive predictive value (PPV) plus anatomical lesion co-localization. The trial demonstrated that 18F-DCFPyL met the primary endpoint, showing effective disease localization in cases where conventional imaging was negative, irrespective of PSA levels (CLR was 73.3% for PSA <0.5 ng/mL and similar for PSA 0.5-1.0 ng/mL). However, detection rates were lower at lower PSA levels; for instance, patients with a PSA <0.5 ng/mL had a detection rate of 36.2%, compared to 66.7% in those with a PSA between 1.0 and 2.0 ng/mL. Notably, almost two-thirds (n=131; 63.9%) of the 205 patients experienced a change in their intended disease management based on the results of the PSMA PET. (4)

The proPSMA study was a multi-center, two-arm randomized controlled trial among men with histologically confirmed prostate cancer who were being considered for curative intent radical prostatectomy or radiotherapy. To be eligible for inclusion, men must have had at least one high-risk factor including prostate-specific antigen (PSA) greater than or equal to 20 ng/mL, ISUP grade group 3-5, or clinical stage T3 or greater. After enrollment, patients were randomly assigned in a 1:1 ratio to either conventional imaging performed using bone scan and CT or PSMA PET/CT. The trial enrolled 302 patients across 10 sites and reported that PSMA PET/CT outperforms conventional imaging for staging patients with high-risk prostate cancer. PSMA PET-CT had a 27% absolute greater AUC for accuracy compared to conventional imaging (95% confidence interval [CI] 23-31): 92% (95% CI 88-95%) vs. 65% (60-69%) and conventional imaging had both a lower sensitivity (38% vs. 85%) and also a lower specificity (91% vs. 98%). (5)
The comparison between PSMA PET and choline PET was conducted in the PYTHON study, a prospective, open-label, cross-over, comparative trial with randomized administration of 18F-DCFPyL (investigational medicinal product) or 18F-fluoromethylcholine (choline). This study enrolled men with rising prostate-specific antigen (PSA) levels after initial curative therapy. The primary objective was to compare the per-patient detection rates of 18F-DCFPyL versus choline PET. Detection rates were defined as the percentage of positive PET/CT scans identified by three central imaging readers. The trial enrolled 205 patients with first biochemical recurrence after radical prostatectomy (73%) or radiation therapy (27%). The per-patient detection rate was significantly higher for 18F-DCFPyL compared to 18F-fluoromethylcholine PET/CTs (58% vs. 40%, p < 0.0001). Notably, detection rates increased with higher PSA values for both radiotracers (PSA ≤ 0.5 ng/mL: 35% vs. 30%).(6)
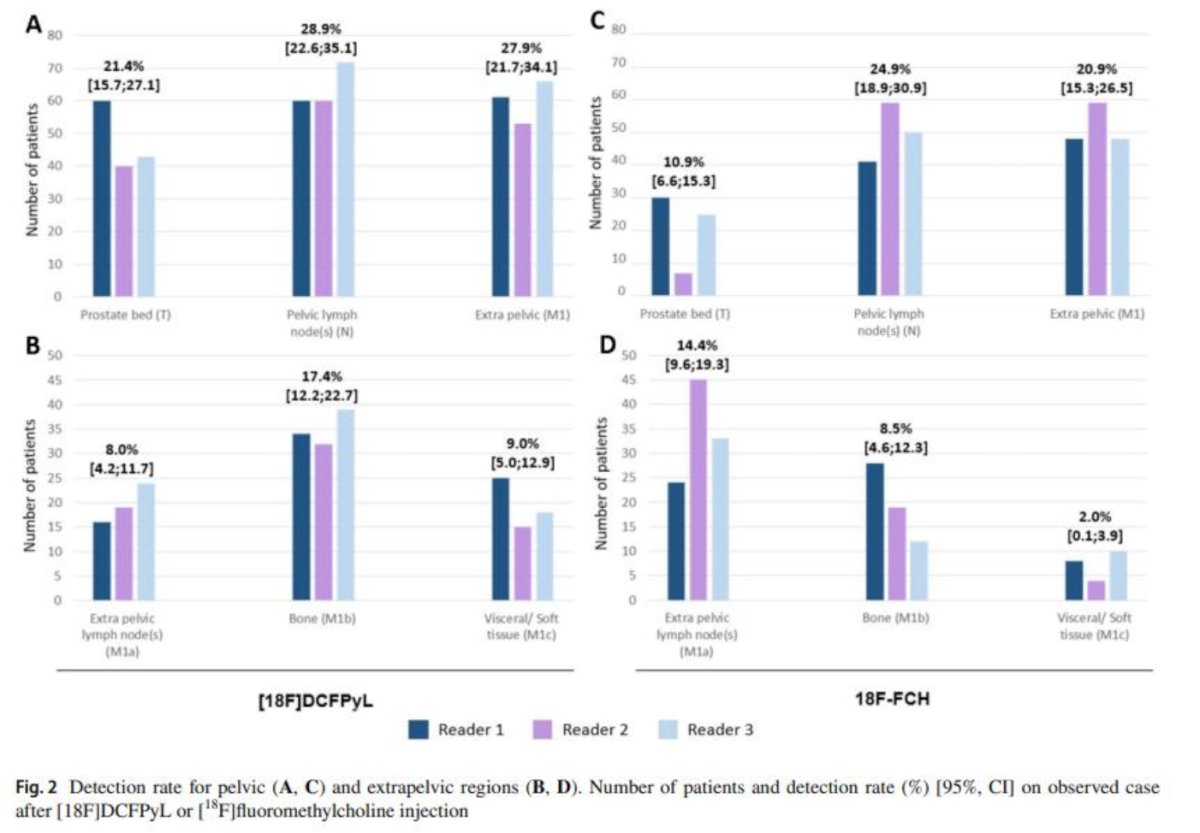
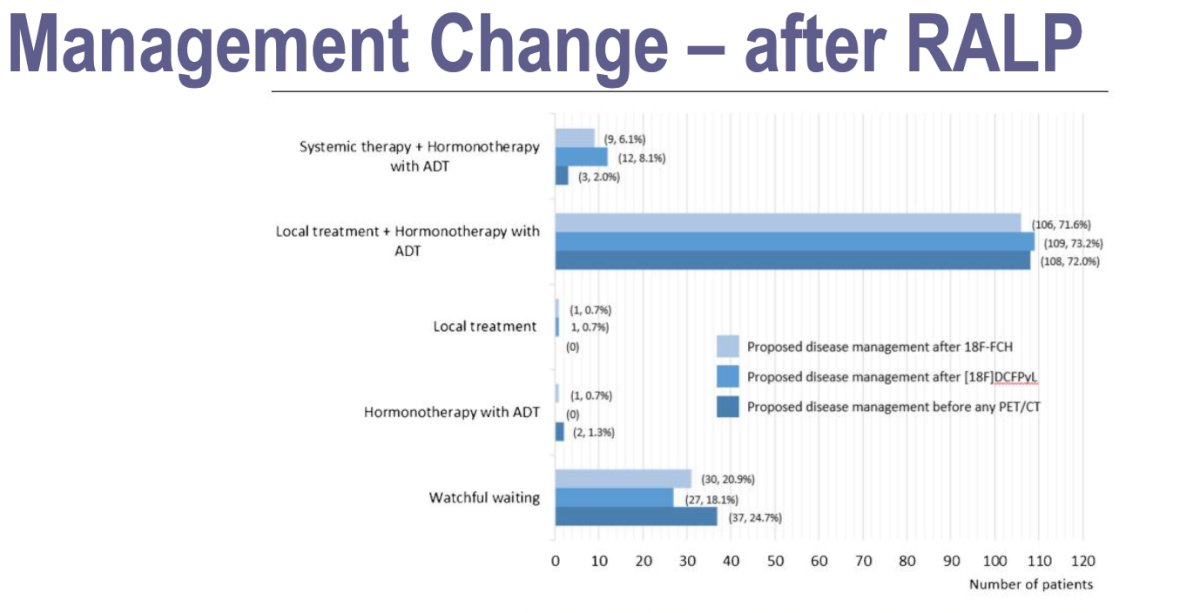
18F-DCFPyL PET/CT had an impact on PM in 44% of patients versus 29% for 18F-fluoromethylcholine. The management change in patients initially treated with robot assisted laparoscopic prostatectomy (RALP) proposed by the truth panel (multidisciplinary independent board) was 73.2% local treatment + ADT according to 18F-DCFPyL and 71.6% according to the 18F-fluoromethylcholine. Local treatment change was the same for both tracers (0.7%).
Similarly, the change in management for patients initially treated with curative radiation therapy was significant. Initially, 60% of these patients were proposed to undergo androgen deprivation therapy (ADT) before any PET/CT. After 18F-DCFPyL PET/CT, this recommendation was reduced to 29.1%, while after 18F-fluoromethylcholine PET/CT, it was reduced to 27.8%. Additionally, the change from the proposed systemic therapy before any PET/CT (7.3%) was substantial, with 34.5% of patients experiencing a change after 18F-DCFPyL PET/CT and 19.6% after 18F-fluoromethylcholine PET/CT.

Dr. Oprea-Lager reported that the PYTHON trial demonstrated a higher per-patient detection rate with 18F-DCFPyL PSMA PET/CT compared to 18F-choline PET/CT for biochemical recurrent prostate cancer. The 18F-DCFPyL PSMA PET/CT exhibited significantly greater per-patient sensitivity compared to 18F-choline PET/CT. Additionally, 18F-DCFPyL PSMA PET/CT impacted patient management in 44% of cases and was both safe and well tolerated.
The impact of PSMA PET on management changes in biochemical recurrence (BCR) is a key area of study in the PSMA PET era. A study using 68Ga-PSMA-11 documented a management change in 53% of patients with BCR prostate cancer. Similarly, a retrospective study involving 253 patients with BCR who underwent restaging with 18F-DCFPyL PET/CT found a management change in 41% of cases. (7) However, there remains a lack of data confirming whether enhanced imaging and management change improves oncological outcomes for the entire group of patients with BCR. There is ongoing discussion about whether this stage migration might be related to the Will Rogers phenomenon, where improved outcomes in PSMA-guided salvage therapy groups could be due to better patient selection.
Dr. Oprea-Lager emphasized the importance of standardizing PSMA PET reporting and presented the updated PSMA PET/CT Joint EANM Procedure Guideline/SNMMI Procedure Standard for prostate cancer imaging, version 2.0. This guideline aims to assist physicians in recommending, acquiring, interpreting, and reporting the results of PSMA ligand PET/CT for the initial diagnosis, staging, and restaging of prostate cancer. It also provides guidance on patient selection, PET/CT acquisition, image interpretation, and the preparation of clinical reports. The new standard incorporates the use of the molecular imaging (mi) classification. (8)
Dr Oprea-Lager concluded her presentation with the following takeaways:
- PSMA PET/CT is a game changer for PCa after primary treatment
- It offers superior accuracy in comparison to conventional imaging and previous tracers (Choline) used for PCa diagnosis
- PSMA PET/CT has a high impact on patient management, while being safe and well tolerated
- Reporting of PSMA PET should follow dedicated imaging guidelines (EANM/SNMMI) and standardization is critical to improve reproducibility.
Presented by: Daniela-Elena Oprea-Lager, MD, PhD, is Associate Professor of Nuclear medicine at the Department of Radiology and Nuclear medicine, Amsterdam University Medical Centers (UMC).
Written by: Julian Chavarriaga, MD –Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 European Society of Medical Oncology (ESMO) Annual Meeting, Barcelona, Spain, Fri, Sept 13 – Tues, Sept 17, 2024.
References:
- Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N, Gandaglia G, Gillessen S, Grivas N, Grummet J, Henry AM, van der Kwast TH, Lam TB, Lardas M, Liew M, Mason MD, Moris L, Oprea-Lager DE, van der Poel HG, Rouvière O, Schoots IG, Tilki D, Wiegel T, Willemse PM, Cornford P. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021 Feb;79(2):243-262. doi: 10.1016/j.eururo.2020.09.042. Epub 2020 Nov 7. PMID: 33172724.
- Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, Bolton D, Lawrentschuk N. Sensitivity, Specificity, and Predictors of Positive 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016 Dec;70(6):926-937. doi: 10.1016/j.eururo.2016.06.021. Epub 2016 Jun 28. PMID: 27363387.
- Pienta KJ, Gorin MA, Rowe SP, Carroll PR, Pouliot F, Probst S, Saperstein L, Preston MA, Alva AS, Patnaik A, Durack JC, Stambler N, Lin T, Jensen J, Wong V, Siegel BA, Morris MJ. A Phase 2/3 Prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in Prostate Cancer Patients (OSPREY). J Urol. 2021 Jul;206(1):52-61. doi: 10.1097/JU.0000000000001698. Epub 2021 Feb 26. PMID: 33634707; PMCID: PMC8556578.
- Morris MJ, Rowe SP, Gorin MA, Saperstein L, Pouliot F, Josephson D, Wong JYC, Pantel AR, Cho SY, Gage KL, Piert M, Iagaru A, Pollard JH, Wong V, Jensen J, Lin T, Stambler N, Carroll PR, Siegel BA; CONDOR Study Group. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase III, Multicenter Study. Clin Cancer Res. 2021 Jul 1;27(13):3674-3682. doi: 10.1158/1078-0432.CCR-20-4573. Epub 2021 Feb 23. PMID: 33622706; PMCID: PMC8382991.
- Hofman, Michael S., Nathan Lawrentschuk, Roslyn J. Francis, Colin Tang, Ian Vela, Paul Thomas, Natalie Rutherford et al. "Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multi-centre study." The Lancet (2020).
- Oprea-Lager DE, Gontier E, García-Cañamaque L, Gauthé M, Olivier P, Mitjavila M, Tamayo P, Robin P, García Vicente AM, Bouyeure AC, Bailliez A, Rodríguez-Fernández A, Mahmoud SB, Vallejo-Casas JA, Maksud P, Merlin C, Blanc-Durand P, Drouet C, Tissot H, Vierasu I, Vander Borght T, Boos E, Chossat F, Hodolic M, Rousseau C. [18F]DCFPyL PET/CT versus [18F]fluoromethylcholine PET/CT in Biochemical Recurrence of Prostate Cancer (PYTHON): a prospective, open label, cross-over, comparative study. Eur J Nucl Med Mol Imaging. 2023 Sep;50(11):3439-3451. doi: 10.1007/s00259-023-06301-5. Epub 2023 Jun 21. PMID: 37341747; PMCID: PMC10542307.
- Meijer D, van Leeuwen PJ, Oosterholt PMJ, Bodar YJL, van der Poel HG, Hendrikse NH, Donswijk ML, Wondergem M, Vellekoop AE, van Moorselaar RJA, Nieuwenhuijzen JA, Oprea-Lager DE, Vis AN. Management impact of 18F-DCFPyL PET/CT in hormone-sensitive prostate cancer patients with biochemical recurrence after definitive treatment: a multicenter retrospective study. Eur J Nucl Med Mol Imaging. 2021 Aug;48(9):2960-2969. doi: 10.1007/s00259-021-05222-5. Epub 2021 Feb 5. PMID: 33547552; PMCID: PMC8263452.
- Fendler WP, Eiber M, Beheshti M, Bomanji J, Calais J, Ceci F, Cho SY, Fanti S, Giesel FL, Goffin K, Haberkorn U, Jacene H, Koo PJ, Kopka K, Krause BJ, Lindenberg L, Marcus C, Mottaghy FM, Oprea-Lager DE, Osborne JR, Piert M, Rowe SP, Schöder H, Wan S, Wester HJ, Hope TA, Herrmann K. PSMA PET/CT: joint EANM procedure guideline/SNMMI procedure standard for prostate cancer imaging 2.0. Eur J Nucl Med Mol Imaging. 2023 Apr;50(5):1466-1486. doi: 10.1007/s00259-022-06089-w. Epub 2023 Jan 5. PMID: 36604326; PMCID: PMC10027805.


