Introduction
Metastatic urothelial carcinoma is associated with a poor prognosis, with a median overall survival of less than two years. To date, combination platinum-based chemotherapy remains the standard of care first line treatment for these patients who are suitable for chemotherapy. This Center of Excellence article will assess criteria for determining cisplatin chemotherapy eligibility, review the landmark trials that established cisplatin-based chemotherapy as the standard of care for first-line treatment, and review recent data for avelumab maintenance therapy among patients that did not progress on first-line chemotherapy.
Determining Cisplatin Eligibility
Determining cisplatin eligibility is important for medical oncologists planning treatment for patients diagnosed with metastatic urothelial carcinoma. One method to do so is using the Galsky criteria, which has become a common modality for determining eligibility for cisplatin-based chemotherapy.1 Based on a review of phase II and phase III trials, Galsky and colleagues determined that unfit patients for cisplatin-based chemotherapy meet at least one of the following criteria:- Eastern Cooperative Oncology Group performance status of 2
- Creatinine clearance <60 mL/min
- Grade ≥ 2 hearing loss
- Grade ≥ 2 neuropathy
- New York Heart Association Class III heart failure
First-Line Cisplatin-Based Chemotherapy: MVAC, Gemcitabine + Cisplatin, and Dose Dense MVAC
The MVAC regimen was first reported in 1985 at Memorial Sloan Kettering Cancer Center to show sensitivity to urothelial carcinoma.2 Patients with measurable disease had a 72% response rate and 36% of patients achieved a complete response, with an overall survival in the entire population of 13.1 months.3 In an update of this cohort published in 1999, median overall survival was 14.8 months, with a 5-year survival rate of 17%.4
In 2000, van der Maase et al.5 published a sentinel phase 3 randomized, multinational, multicenter trial that compared gemcitabine + cisplatin versus MVAC. This trial included 405 patients with stage IV urothelial carcinoma and no prior systemic therapy who were randomized to gemcitabine 1,000 mg/m2 days 1, 8, and 15 + cisplatin 70 mg/m2 (n = 203) or standard MVAC every 28 days (n = 202) for a maximum of six cycles. Overall survival was similar in both arms (HR 1.04, 95% CI 0.82 – 1.32):
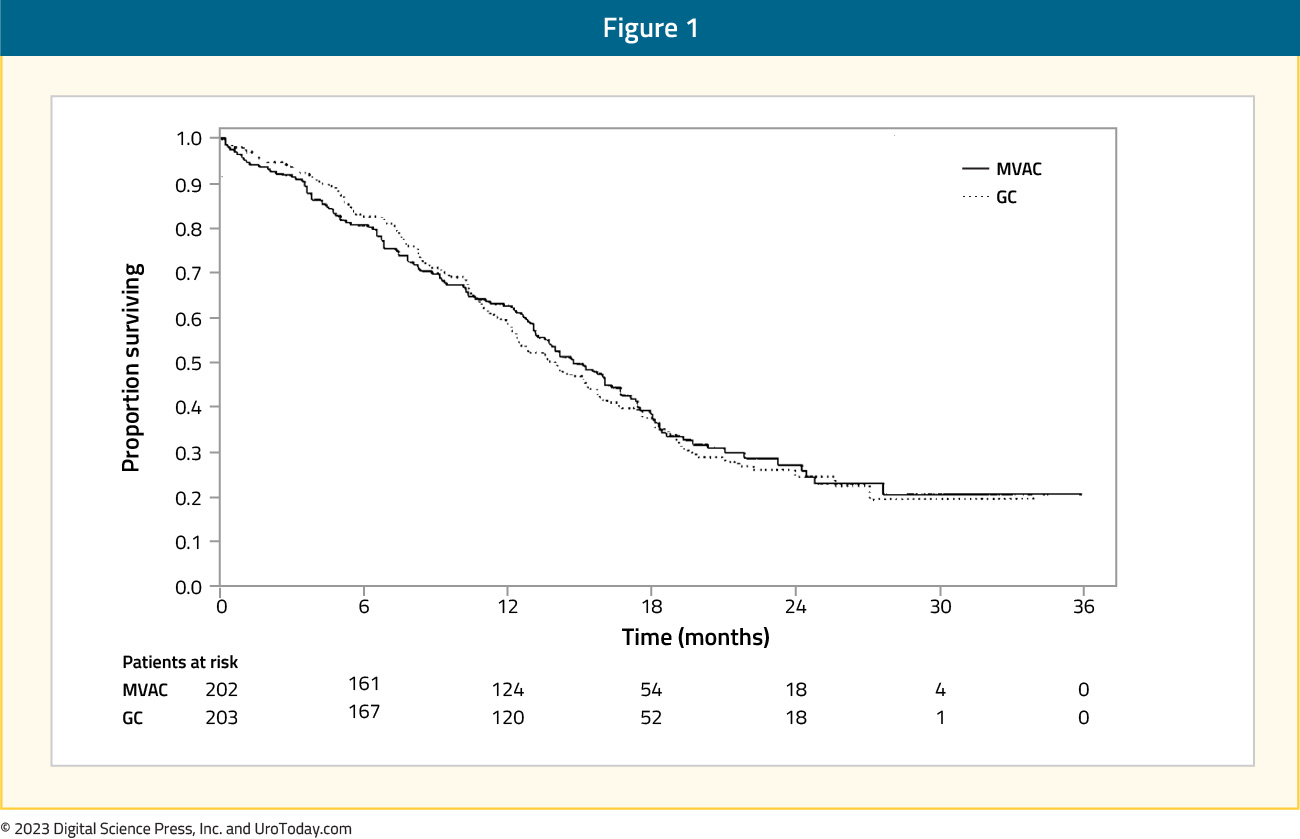
Additionally, there was no difference in time to progressive disease (HR 1.05, 95% CI 0.85 – 1.30), time to treatment failure (HR 0.89, 95% CI 0.72 – 1.10), and response rate (gemcitabine + cisplatin: 49% vs MVAC: 46%). Grade 3-4 anemia (27% versus 18%) and thrombocytopenia (57% versus 21%) occurred more commonly in gemcitabine + cisplatin-treated patients compared to MVAC; however, MVAC patients had higher rates of grade 3-4 neutropenia (82% versus 71%), neutropenic fever (14% versus 2%), and neutropenic sepsis (12% versus 1%). As such, this trial was able to conclude that gemcitabine + cisplatin provides a similar survival advantage to MVAC, with a better-risk benefit ratio.
In an attempt to improve upon the results obtained with MVAC, Dr. Sternberg and colleagues initiated the EORTC 30924 trial assessing dose dense MVAC + recombinant human granulocyte colony-stimulating (GCF) versus classic MVAC as first line treatment for metastatic urothelial carcinoma.6 The trial design for EORTC 30924 is as follows:

Overall, there were 263 patients randomized to 2 weeks of dose dense MVAC versus 4 weeks of MVAC, and with a median follow-up of 38 months, the complete response rate in the dose dense MVAC arm was 21% and partial response rate was 41%, for an overall response rate of 62%. This was superior to the MVAC arm which had a complete response rate of 9% (p = 0.009) and partial response rate was 41%, for an overall response rate of 50% (p = 0.06). Although there was an improvement in progression-free survival among patients receiving dose dense MVAC (HR 0.75, 95% CI 0.58 – 0.98), there was no difference in overall survival (p = 0.122) or time to progression (p = 0.114).
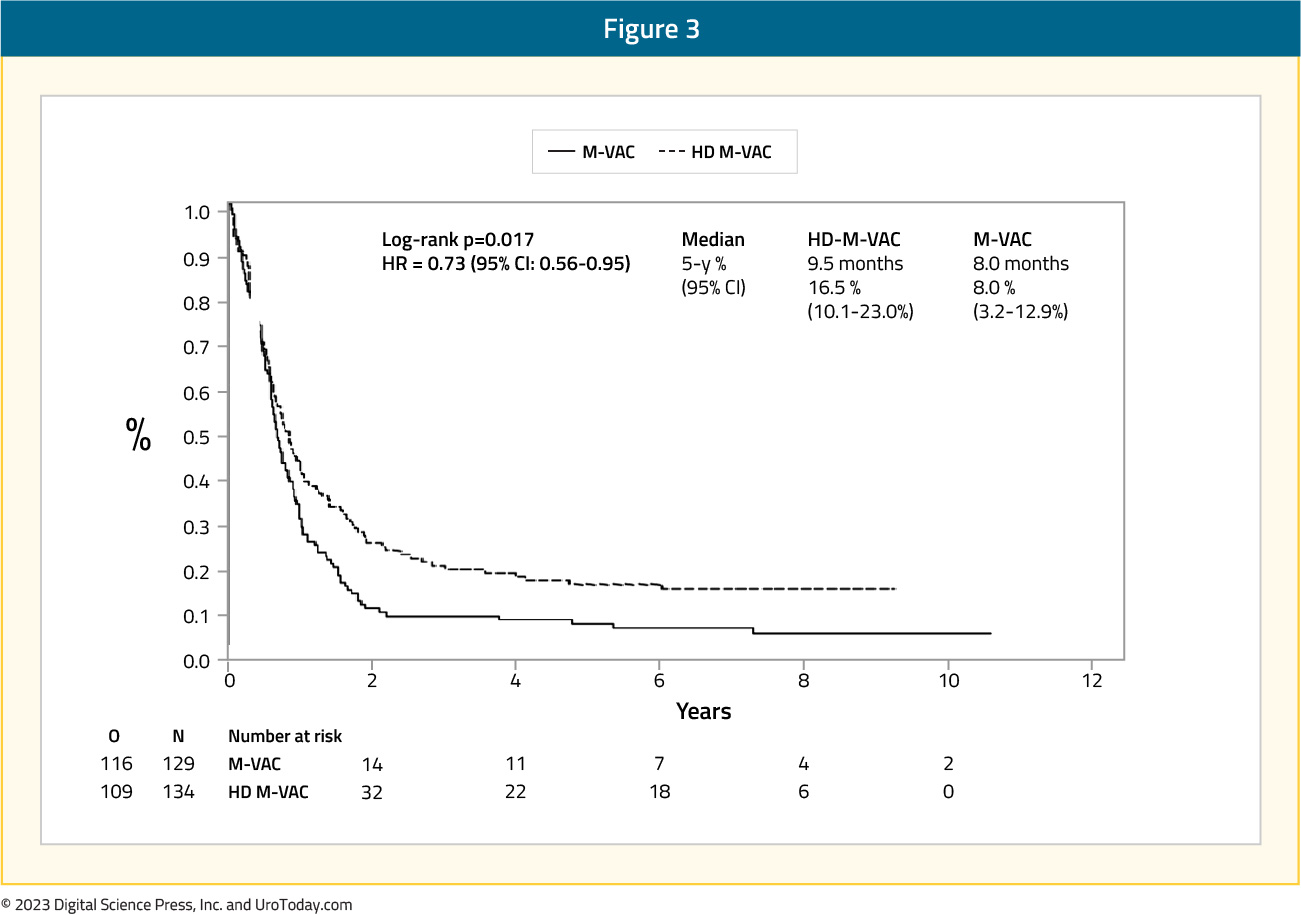
Extended follow-up (median 7.3 years in both groups) of the EORTC 30924 trial was published in 2006.7 At the time of this analysis, 24.6% of patients were alive in the dose dense MVAC arm versus 13.2% in the MVAC arm. The median progression-free survival was still improved in the dose dense MVAC arm (9.5 months) compared to the MVAC (8.1 months) arm (HR 0.73, 95% CI 0.56 – 0.95):
Additionally, the median overall survival was 15.1 months in the dose dense MVAC arm compared to 14.9 months in the MVAC arm (HR 0.76, 95% CI 0.58 – 0.99), with a 5-year survival rate of 21.8% and 13.5%, respectively:
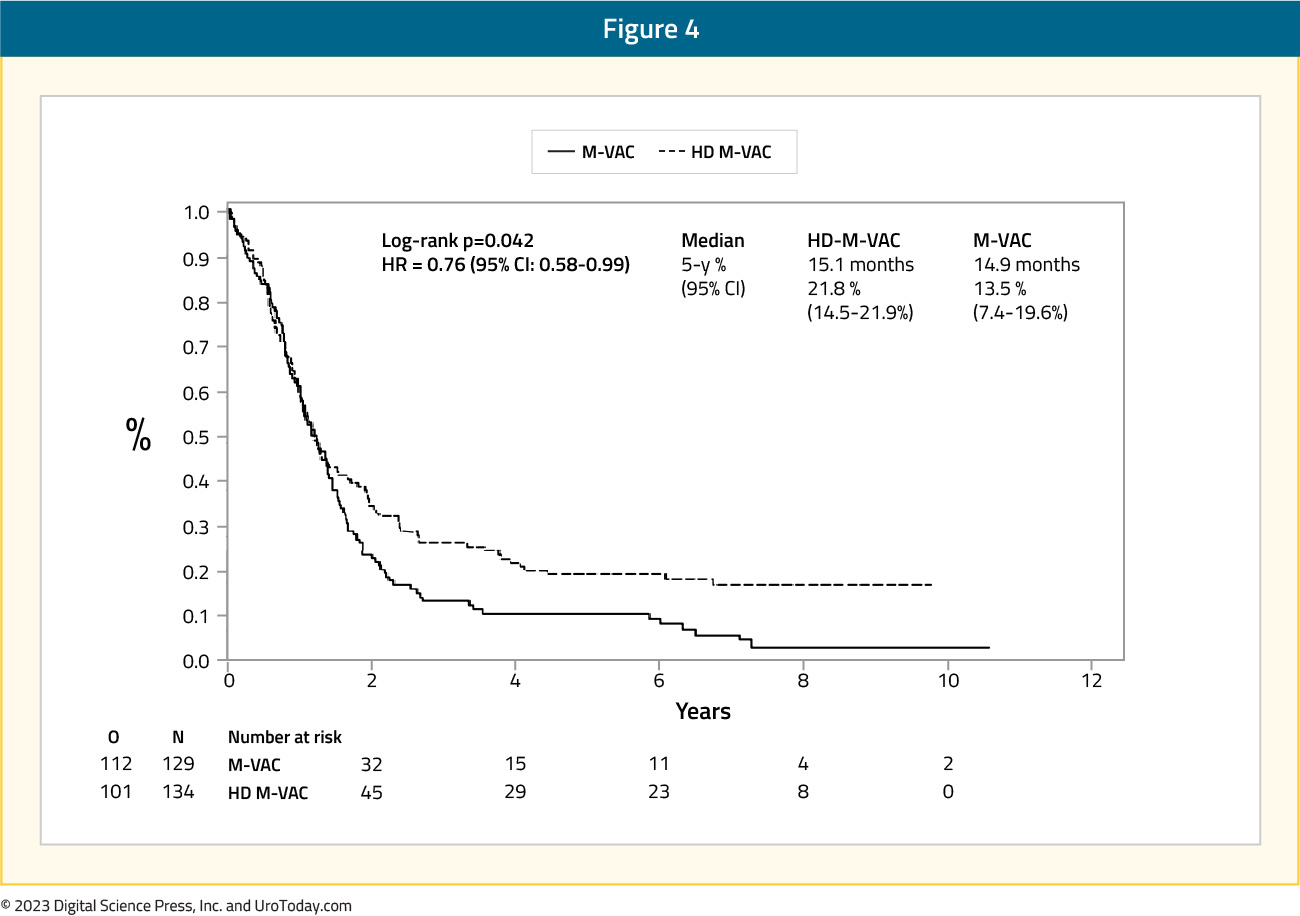
There was one death from toxicity in each arm, and more patients died secondary to malignant disease in the MVAC arm (76%) compared to those in the dose dense MVAC arm (64.9%). With this longer follow-up for the EORTC 30924 trial, initial results were confirmed showing that dose dense MVAC had a borderline statistically significant relative reduction in the risk of progression and death compared to MVAC.
In one of the largest real world evidence assessments of gemcitabine + cisplatin versus MVAC chemotherapy for cisplatin-eligible patients, Lee and colleagues used the Korean National Health Insurance Service database from 2004 to 2016.8 This cohort consisted of 3,108 patients, of which 2,880 received gemcitabine + cisplatin and 228 received MVAC. The use of gemcitabine + cisplatin was the highest in 2013-2016 (37.5%), whereas that of MVAC was highest in 2004-2008 (39.5%). Overall, the 5-year survival rate was 23.4% for the gemcitabine + cisplatin group and 25.0% for the MVAC group, including a median overall survival of 39.6 months and 37 months, respectively:
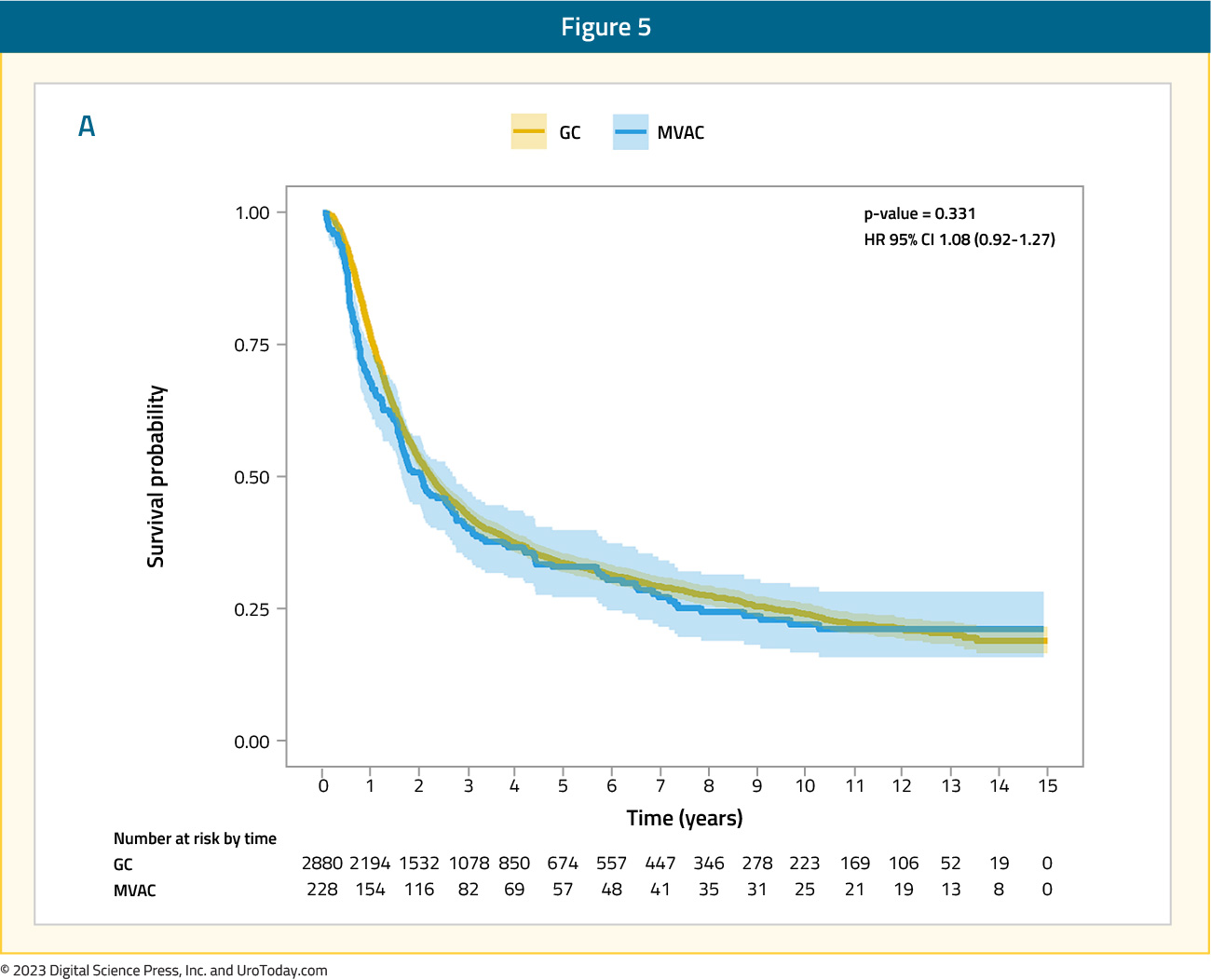
Interestingly, a subgroup analysis revealed that a period of >= 3 months from diagnosis to systemic therapy enhanced the prognostic effects of the gemcitabine + cisplatin regimen (HR 1.35, 95% CI 1.04 – 1.74).
Avelumab Maintenance Therapy
Platinum-based chemotherapy generally shows an objective response in 40-50% of patients and disease control in 75-80% of patients; however, most patients have disease progression in 9 months, with a median overall survival of 14-15 months.5-7 As such, there is an unmet clinical need for the treatment of patients who have completed first line chemotherapy (4 – 6 cycles of cisplatin-based chemotherapy) and did not experience disease progression. The JAVELIN Bladder 100 trial was a phase 3 trial that randomly assigned patients with unresectable locally advanced or metastatic urothelial cancer who did not have disease progression with first-line chemotherapy to receive best supportive care with or without maintenance avelumab (10 mg/kg IV every 2 weeks).9 Patients were randomized from May 2016 to June 2019 at 197 sites in 29 countries, with 350 patients in each of the arms. The trial design for JAVELIN Bladder 100 is as follows:
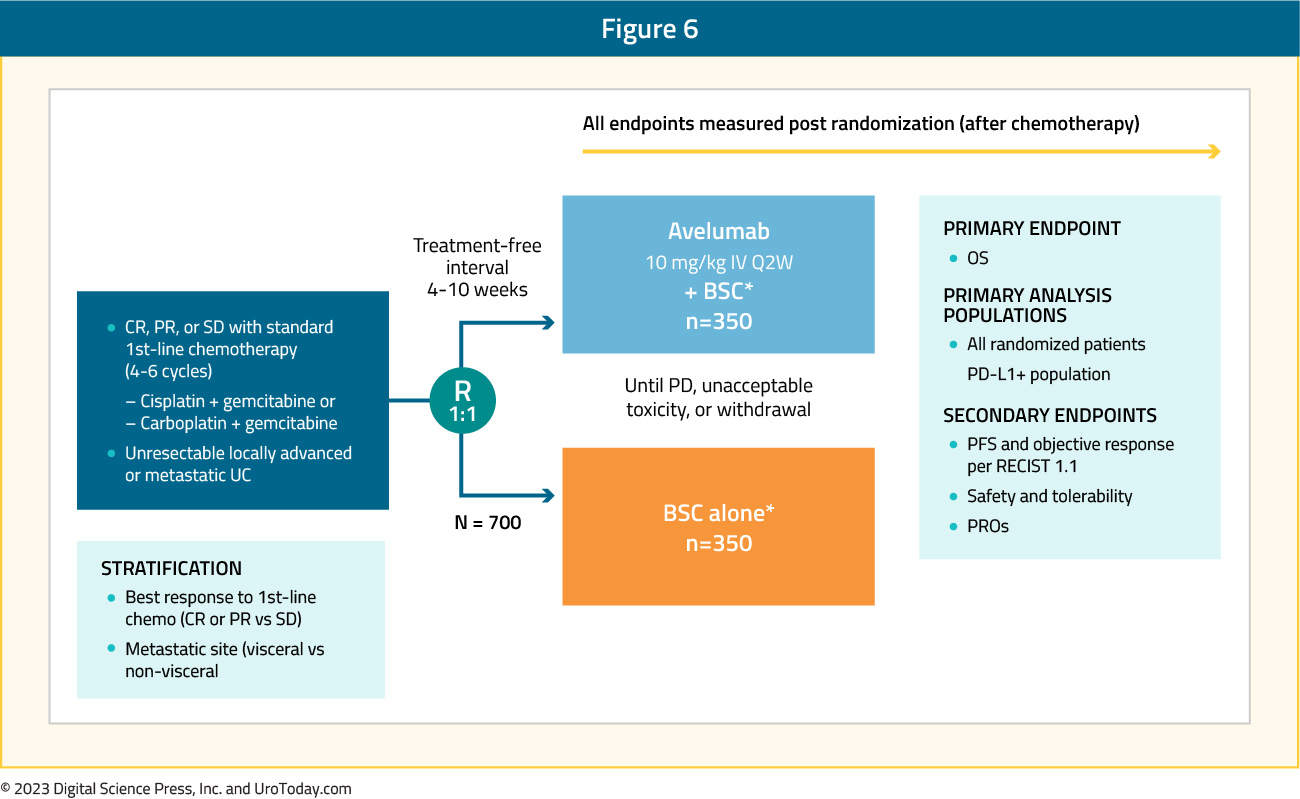
At the time of data cutoff, 24.3% of patients in the avelumab group and 7.4% in the control group were still receiving treatment, with the most common reason for discontinuation being progressive disease (54% in the avelumab group versus 75% in the control group). Furthermore, subsequent anticancer drug therapy was received by 42.3% of patients in the avelumab group compared to 61.7% of patients in the control group.
Overall survival at 1 year was 71.3% (95% CI 66.0 – 76.0) in the avelumab group compared to 58.4% (95% CI 52.7 – 63.7) in the control group, with a median overall survival of 21.4 months and 14.3 months, respectively (HR 0.69, 95% CI 0.56 – 0.86):

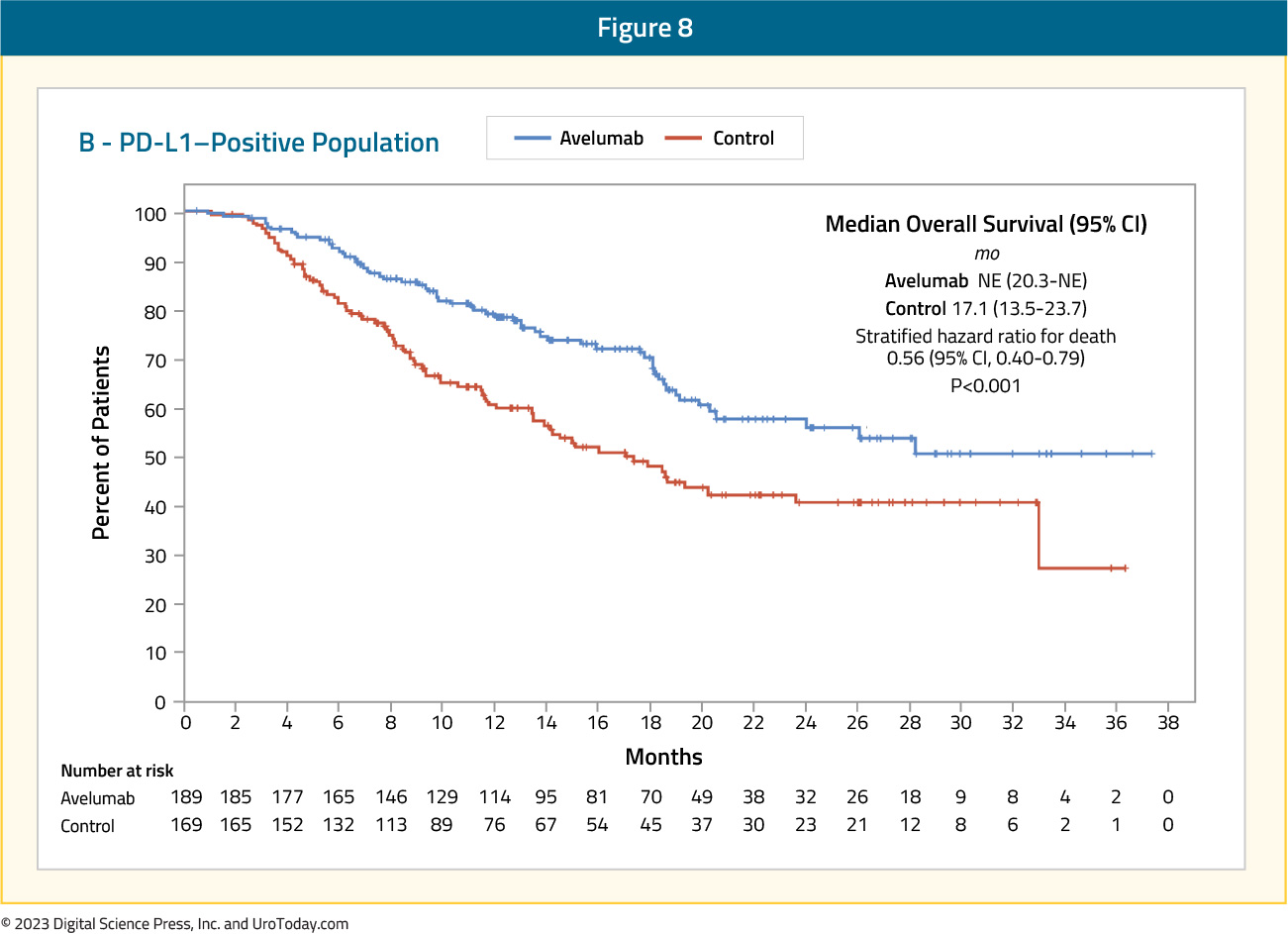
An overall survival benefit favoring avelumab was even more pronounced in the PD-L1 positive population (HR 0.56, 95% CI 0.40 – 0.79):
The key secondary endpoint in this trial was progression free survival, which was also longer in the avelumab group, with a median of 3.7 months (95% CI 3.5 – 5.5) compared to 2.0 months (95% CI 1.9 – 2.7) in the control group (HR 0.62, 95% CI 0.52 – 0.75):

This progression free survival benefit for avelumab was also evident in the PD-L1 positive population (HR 0.56, 95% CI 0.43 – 0.73). Grade 3 or worse adverse events occurred in 47.4% of patients receiving avelumab compared to 25.2% of patients in the control group, with adverse events leading to discontinuation occurring in 11.9% of patients in the avelumab group. The most frequent category of immune-related adverse events was thyroid disorders, which occurred in 12.2% of patients.
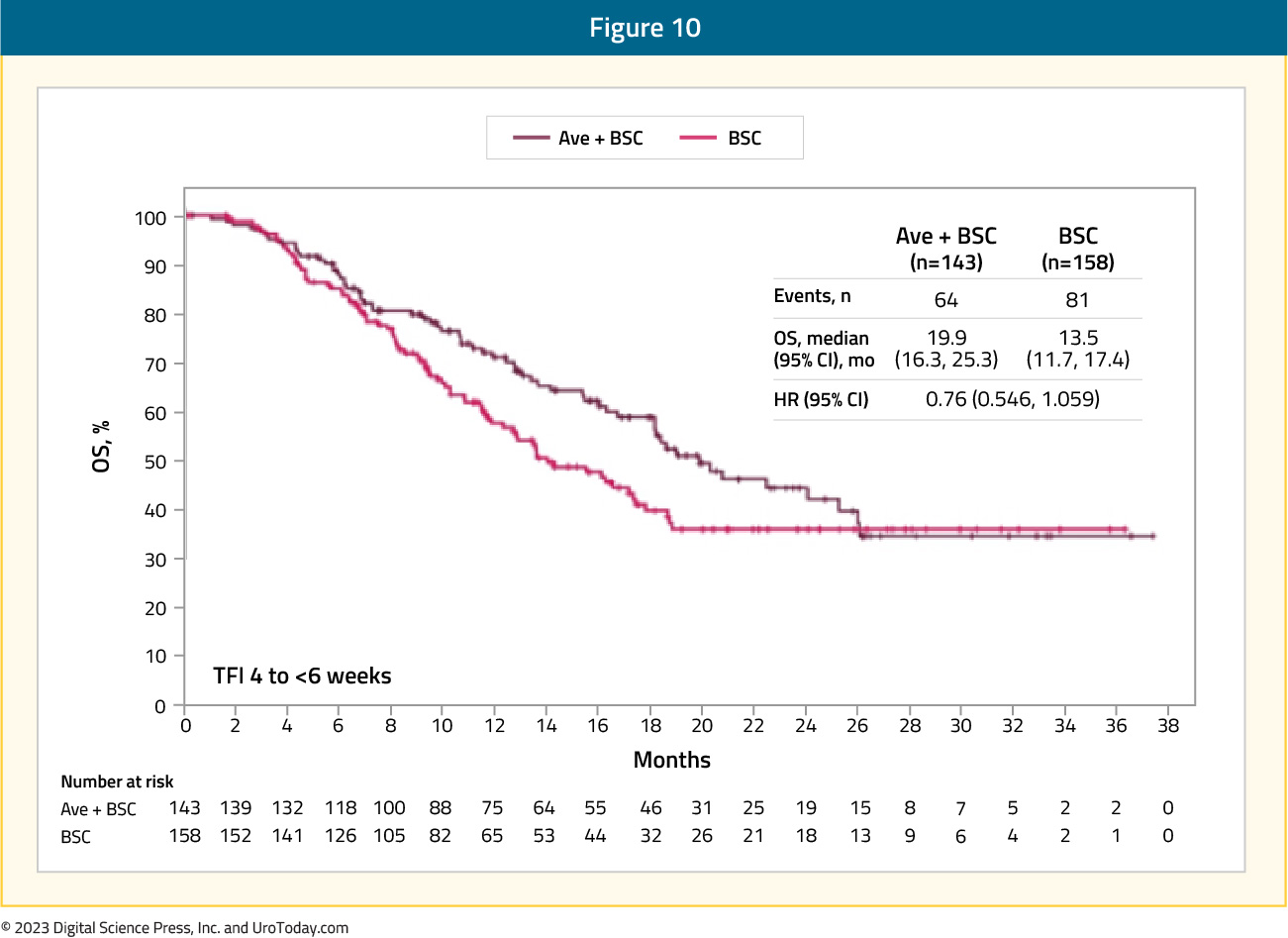
Since the publication of this landmark trial in the New England Journal of Medicine, there have been several additional analyses presented at international conferences. At the ASCO 2021 annual meeting, Dr. Srikala Sridhar presented a subgroup analysis by duration of treatment-free interval from the end of chemotherapy to the start of maintenance. In the avelumab and control group arms, the treatment-free interval was 4 to < 6 weeks in 143 and 158 patients, 6 to < 8 weeks in 109 and 80 patients, and 8 to 10 weeks in 98 and 110 patients, respectively. For both arms combined, however, the treatment-free interval 4 to < 6 weeks subgroup, as compared to the other two subgroups, included significantly more patients with visceral metastases (57.8% vs 54.0% and 50.0%), an objective response with first-line chemotherapy (76.4% vs 69.3% and 68.3%), and an ECOG performance status of 1 (44.5% vs 33.3% and 35.6%). OS was prolonged with avelumab + best supportive care versus best supportive care alone in all subgroups. The HR was 0.76 (95% CI 0.546 - 1.059) in the treatment-free interval 4 to < 6 weeks subgroup (median OS, 19.9 months [95% CI 16.3 - 25.3] vs 13.5 months [95% CI 11.7 - 17.4]):
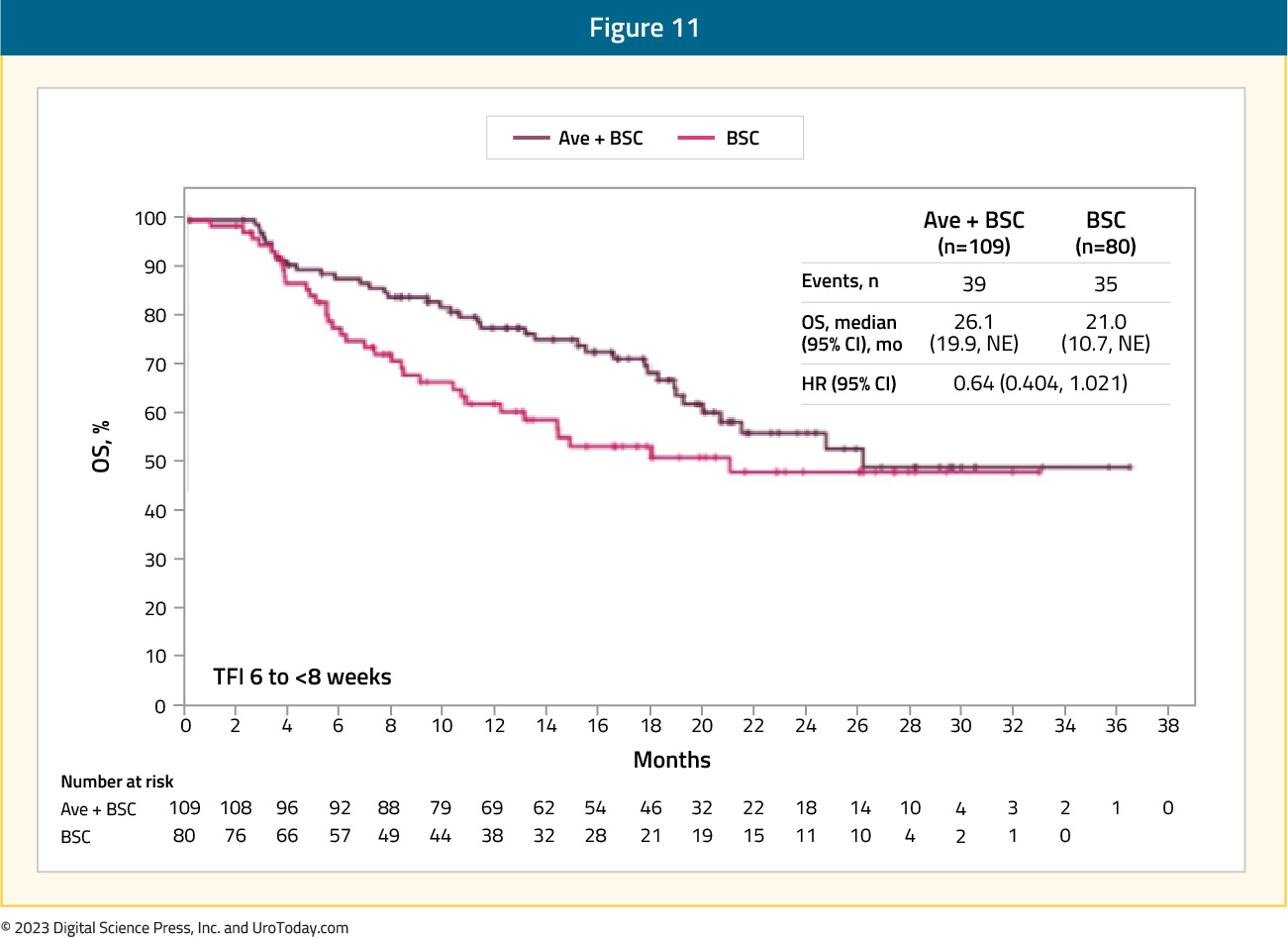
0.64 (95% CI: 0.404, 1.021) in the treatment-free interval 6 to < 8 weeks subgroup (median OS, 26.1 months [95% CI 19.9 to not estimable] vs 21.0 months [95% CI 10.7 to not estimable]):
and 0.70 (95% CI 0.468 - 1.035) in the treatment-free interval 8 to 10 weeks subgroup (median OS, 20.1 months [95% CI 13.8 to not estimable] vs 14.1 months [95% CI 11.7 - 19.6]):

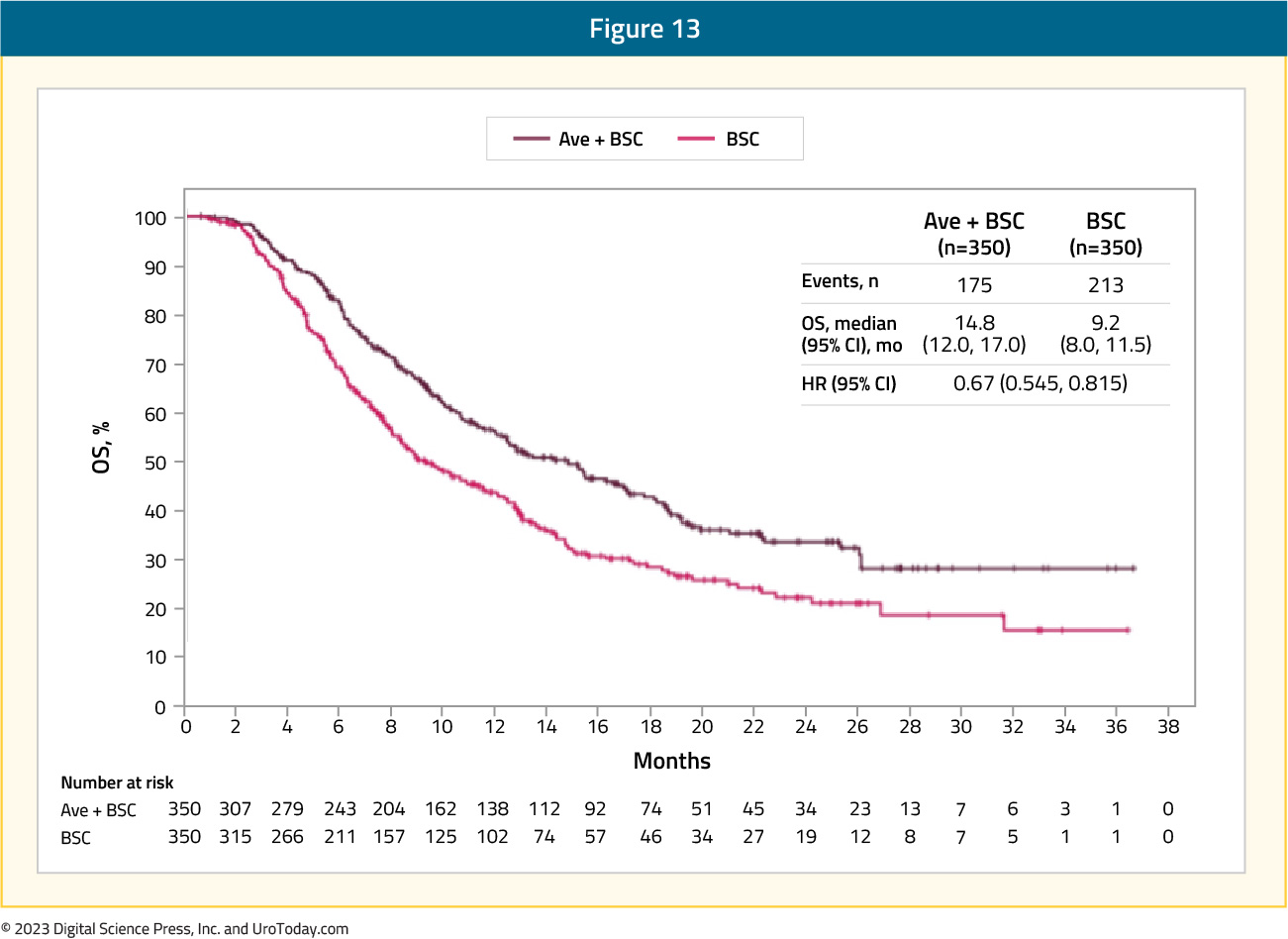
Also presented at ASCO 2021 by Dr. Petros Grivas was a subgroup analysis of time to end of next-line of therapy. Importantly, time to next-line of therapy was analyzed as an alternative to the second progression-free survival (PFS2) endpoint, which could not be analyzed because progression on next-line therapy was not assessed in the trial population. Among all randomized patients, time to end of next-line therapy was prolonged in the avelumab arm (median 14.8 months) versus the control arm (median 9.2 months; HR 0.67, 95% CI 0.545 to 0.815):
Time to end of next-line therapy was also longer in the avelumab + best supportive care arm versus the best supportive care alone arm in patients with PD-L1+ tumors (n = 358) or PD-L1− tumors (n = 270):
- PD-L1+: 18.1 versus 9.0 months, HR 0.61, 95% CI 0.45 - 0.82
- PD-L1-: 11.9 versus 9.3 months, HR 0.76, 95% CI 0.56 - 1.04
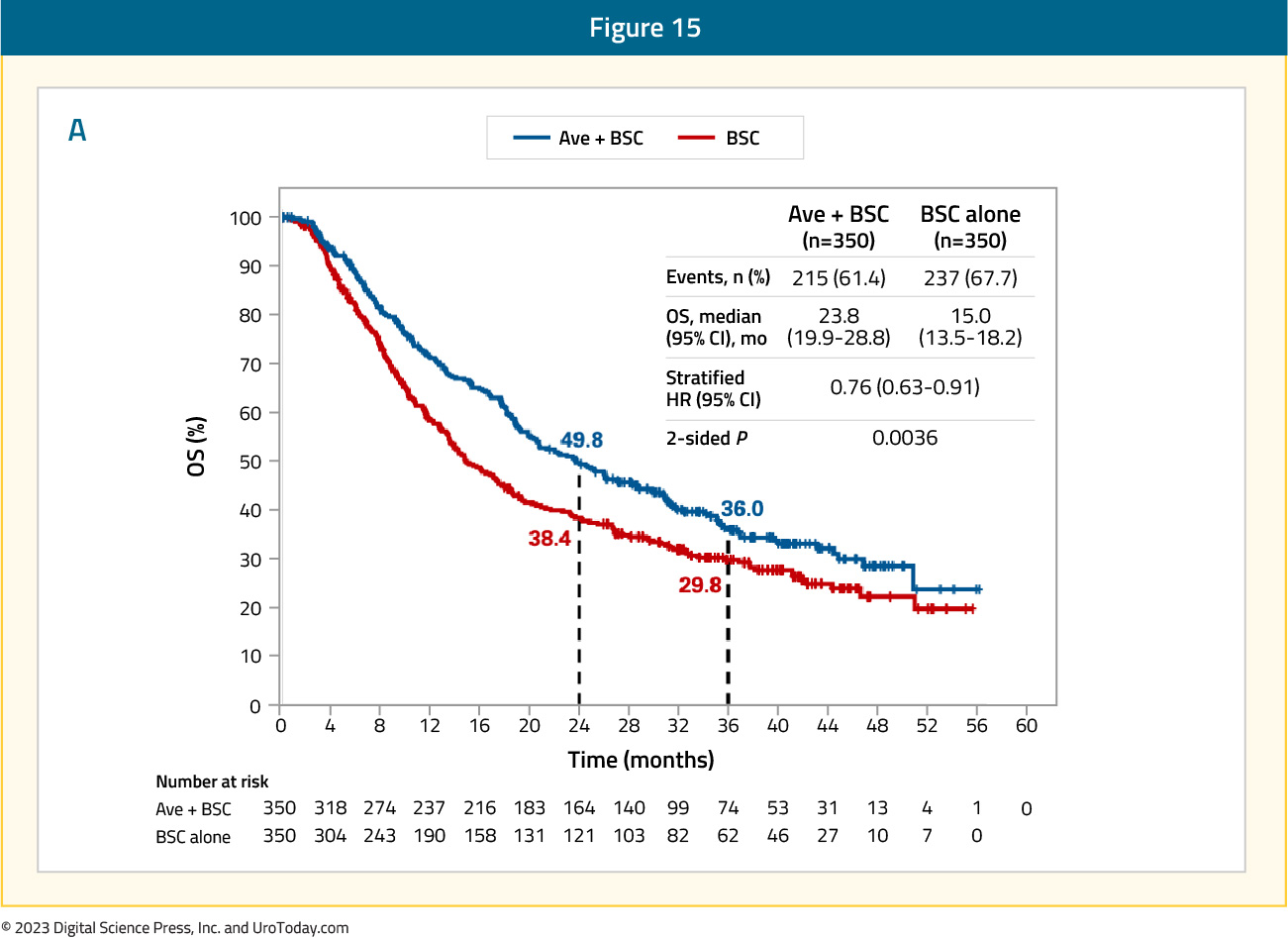
In July 2023, Powles et al.10 published results of the JAVELIN Bladder 100 trial after ≥2 years of follow-up. At the time of the data cutoff for this analysis (June 4, 2021), the median follow-up was 38.0 months for patients receiving avelumab versus 39.6 months for those in the control arm. Of note, 67 (19.5%) patients had received ≥2 years of avelumab maintenance therapy. In this extended analysis, overall survival remained prolonged with avelumab, with a median survival of 23.8 months (95% CI 19.9 – 28.8) versus 15.0 months (95% CI 13.5 – 18.2) for the control arm (HR 0.76, 95% CI 0.63 – 0.91):
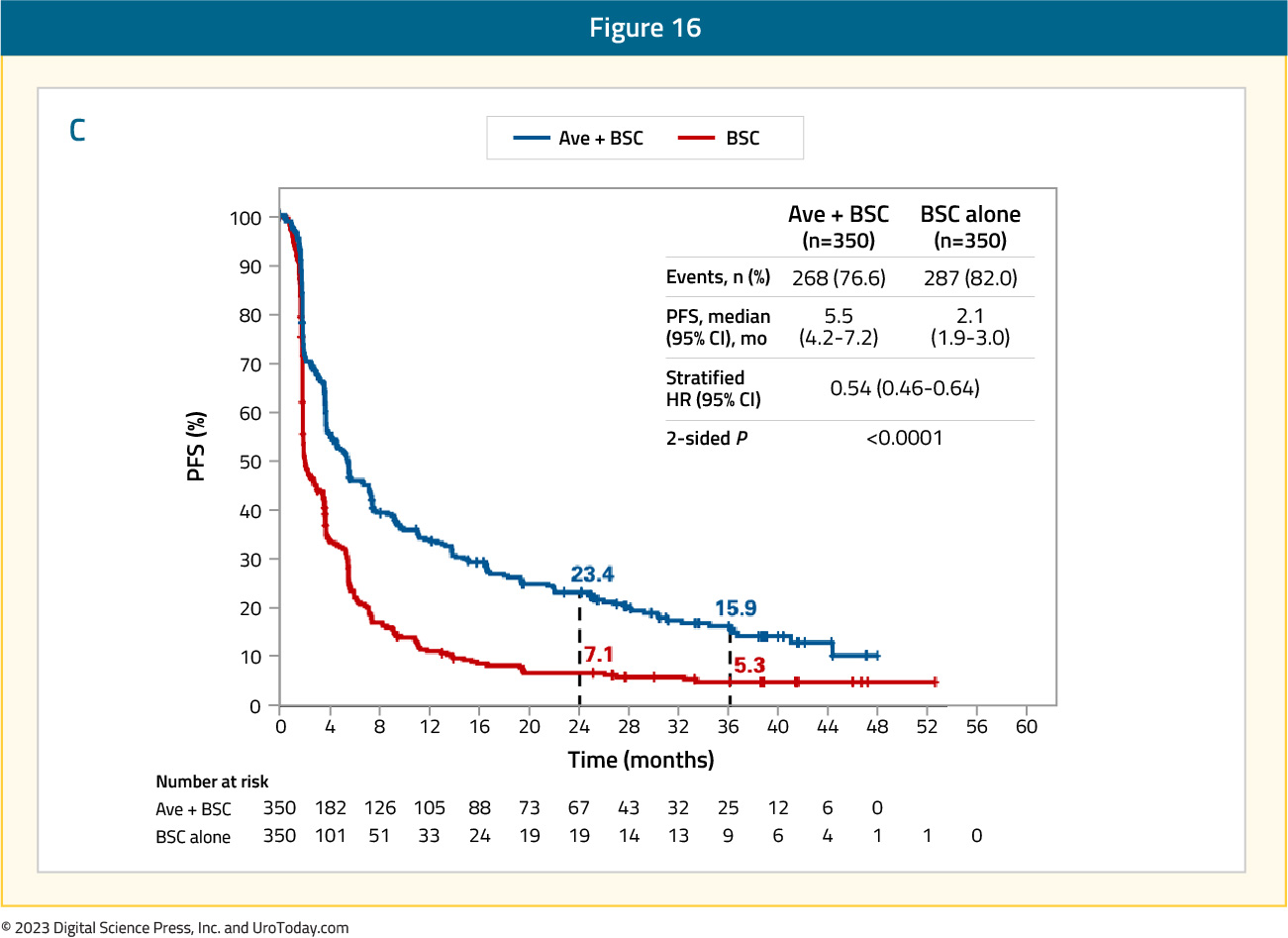
Additionally, investigator-assessed progression free survival remained prolonged for patients receiving avelumab, with a median of 5.5 months (95% CI 4.2 – 7.2) versus 2.1 months (95% CI 1.9 – 3.0) in the control arm (HR 0.54, 95% CI 0.46 – 0.64):
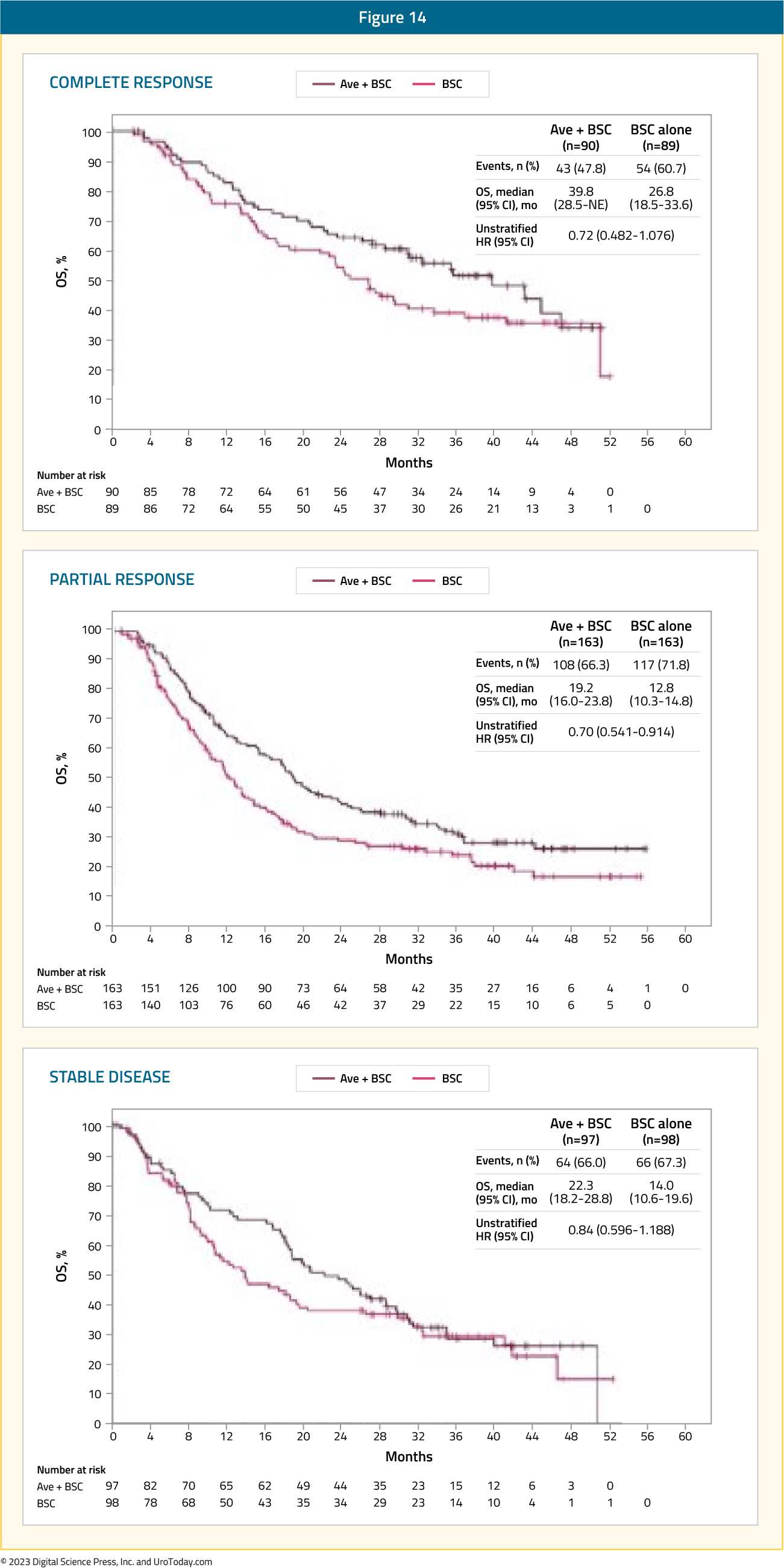
Among responders, the median duration of response was 28.4 months (95% CI 15.9 – 42.3) in the avelumab arm and 26.9 months (95% CI 4.4 to not estimable) in the control arm.
In addition to the efficacy outcomes highlighted above for JAVELIN Bladder 100, this trial also has provided an enriched biomarker population that will hopefully further guide treatment. Powles and colleagues reported that the avelumab survival benefit was associated with PD-L1 expression by tumor cells, tumor mutational burden, APOBEC mutation signatures, expression of genes underlying innate and adaptive immune activity, and the number of alleles encoding high-affinity variants of activating Fcy receptors.11 Although individual biomarkers did not identify patients who may benefit from therapy, this analysis represents new opportunities to enrich patient therapies based on a personalized strategy and inform future clinical trial design.
Conclusions
Despite recent advances in immune checkpoint inhibitor therapy for many malignancies, first-line cisplatin-based chemotherapy remains the standard of care among patients with metastatic urothelial carcinoma who are cisplatin eligible. Depending on disease and/or patient characteristics and/or clinical practice preferences, this may include 4-6 cycles of gemcitabine + cisplatin or dose dense MVAC. Given the efficacy and safety data for avelumab maintenance therapy among patients that did not progress on first-line platinum-based chemotherapy, avelumab maintenance has also become standard of care. Based on early biomarker work from the JAVELIN Bladder 100 trial, we are hopefully closer to personalized treatment approaches and improved efficacy for these patients.Published August 2023


