Introduction
For patients with metastatic urothelial carcinoma, first line therapy for cisplatin eligible patients remains platinum-based chemotherapy1 (followed by maintenance avelumab2), whereas those that are cisplatin ineligible may receive gemcitabine + carboplatin3 (followed by maintenance avelumab2), pembrolizumab,4 or pembrolizumab + enfortumab vedotin.5 Unfortunately, ~50% of patients will eventually have disease progression following first line therapy, which has historically been associated with a dismal prognosis.
Previously used second line therapies, such as paclitaxel, pemetrexed, docetaxel, and vinflunine, have shown limited efficacy, with a median survival of only ~7 months.6-9 Accordingly, there has been a concerted effort over the last few years to assess new treatment options for patients who have progressed after first-line therapy for metastatic urothelial carcinoma. This Center of Excellence Article will explore second-line therapy options for patients, stratified by the class of first line therapy received: (i) platinum-based or other chemotherapy and (ii) immune checkpoint inhibitors.
Second Line Systemic Therapy after Platinum-Based or Other Chemotherapy
The preferred second line regimen for patients following first line platinum-based chemotherapy is pembrolizumab, based on the 2017 results of the KEYNOTE-045 trial.10 In this international, phase 3, open label, randomized clinical trial of 542 patients progressing after platinum-based chemotherapy, pembrolizumab 200 mg every 3 weeks was compared to the investigator’s choice of chemotherapy with paclitaxel, docetaxel, or vinflunine. The trial design for KEYNOTE-045 is as follows:
The co-primary endpoints for KEYNOTE-045 were overall survival and progression-free survival. Over a median follow-up of 14.1 months (range: 9.9 – 22.1), the median duration of treatment was 3.5 months (range: <0.1 – 20.0) for the pembrolizumab group and 1.5 months (range: <0.1 – 14.2) in the chemotherapy group. Overall survival was significantly longer in the pembrolizumab group (median 10.3 months) versus the chemotherapy group (median 7.4 months), with a hazard ratio for death of 0.73 (95% CI: 0.59 – 0.91):

Pembrolizumab was also associated with improved overall survival in patients who had a tumor PD-L1 CPS ≥10%, with a median overall survival of 8.0 months versus 5.2 months in the chemotherapy group (HR: 0.57, 95% CI: 0.37 – 0.88). However, there was no difference in progression free survival between the pembrolizumab group (median 2.1 months) and the chemotherapy group (median 3.3 months; HR: 0.98, 95% CI: 0.81 – 1.19):
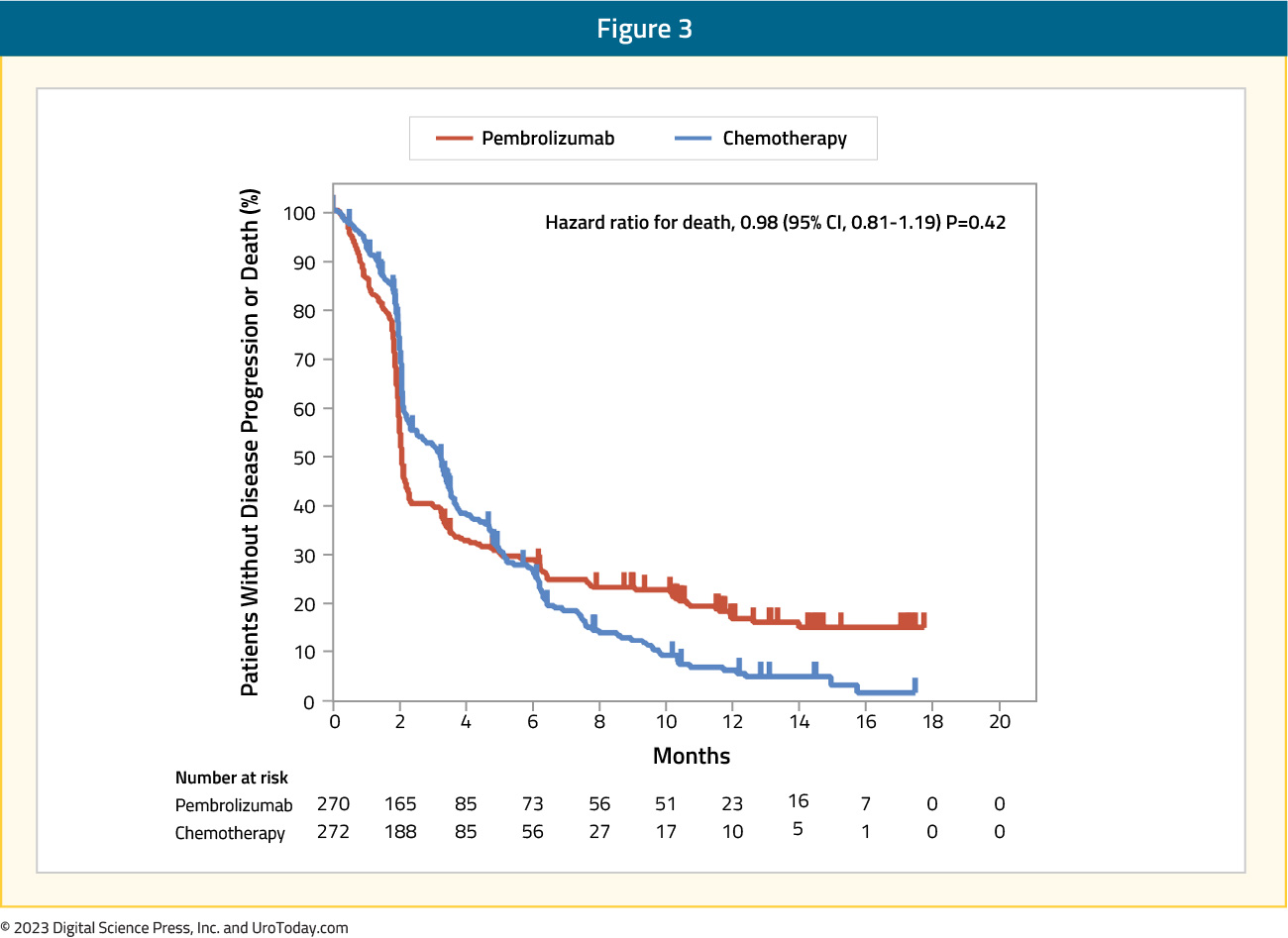
Objective response rate was significantly higher in the pembrolizumab arm (21.1%, 95% CI: 16.4 – 26.5) compared to the chemotherapy arm (11.4%, 95% CI: 7.9 – 15.8; p = 0.001). With regards to adverse events, those that were related to treatment occurred in 61% of patients receiving pembrolizumab compared to 90% of those receiving chemotherapy. Additionally, grade 3-5 adverse events were higher in the chemotherapy arm: 49% versus 15%.
At the 2021 ASCO meeting, Dr. Joaquim Bellmunt presented 5-year follow-up results of the KEYNOTE-045 trial. As of October 1, 2020, the median time from randomization to data cutoff was just over five years. Among patients in the pembrolizumab arm, 9.4% completed two years of therapy compared to 0% of patients in the chemotherapy arm. Importantly, the median overall survival continued to remain longer for patients receiving pembrolizumab versus chemotherapy (10.1 versus 7.2 months; HR: 0.71, 95% CI: 0.59 - 0.86):

Overall survival rates at 48 months were 16.7% for pembrolizumab and 10.1% for chemotherapy, and 14.9% and 8.7%, respectively, at 60 months. Finally, the median duration of response for responders was longer for pembrolizumab versus chemotherapy (29.7 months [1.6+ to 60.5+] versus 4.4 months [1.4+ to 63.1+]), and a greater proportion of responses lasted ≥48 months (40.9% vs 28.3%) and ≥60 months (32.8% vs 28.3%):
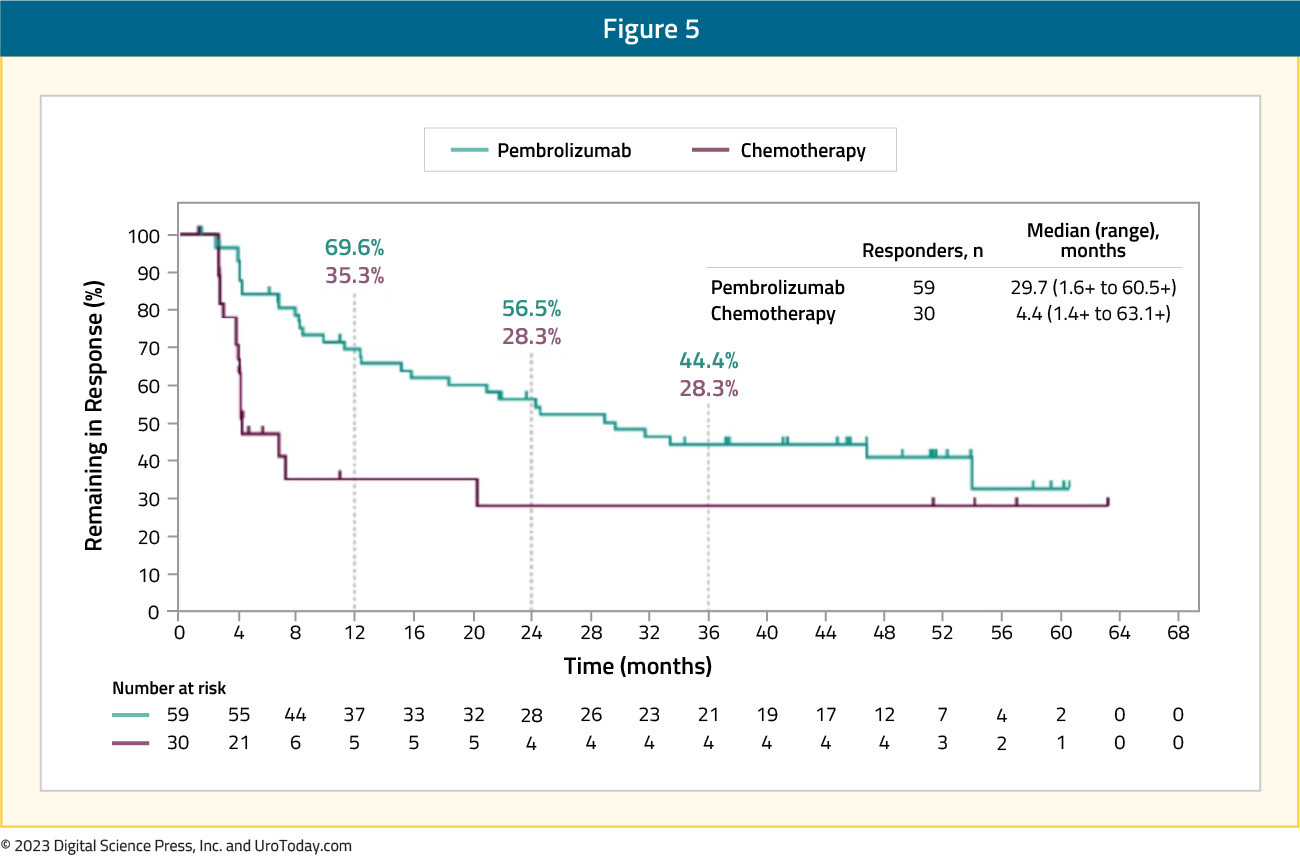
In addition to pembrolizumab, there are other alternative regimens for these patients previously treated with platinum-based chemotherapy, including (i) nivolumab, (ii) avelumab, and (iii) erdafitinib.
The CheckMate 275 trial was a multi-center, single-arm, phase 2 study assessing the activity and safety of nivolumab in patients with metastatic urothelial carcinoma who had progressive disease after platinum-based chemotherapy.11 There were 270 patients that received nivolumab 3 mg/kg every two weeks until disease progression or unacceptable toxicity, with a primary endpoint of objective response in all patients and by tumor PD-L1 expression. CheckMate 275 demonstrated an objective response rate of 19.6% (95% CI 15.0 – 24.9) for nivolumab, with six patients (2%) having a complete response and 46 (17%) having a partial response. Among patients with PD-L1 expression ≥5%, the objective response was 28.4% (95% CI 18.9 – 39.5). At a median follow-up of 7.0 months (IQR 2.96 – 8.77), the median overall survival was 8.7 months (95% CI: 6.1 – not reached) in the overall population and 11.3 months (95% CI: 8.74 – not reached) for those with PD-L1 ≥1%:
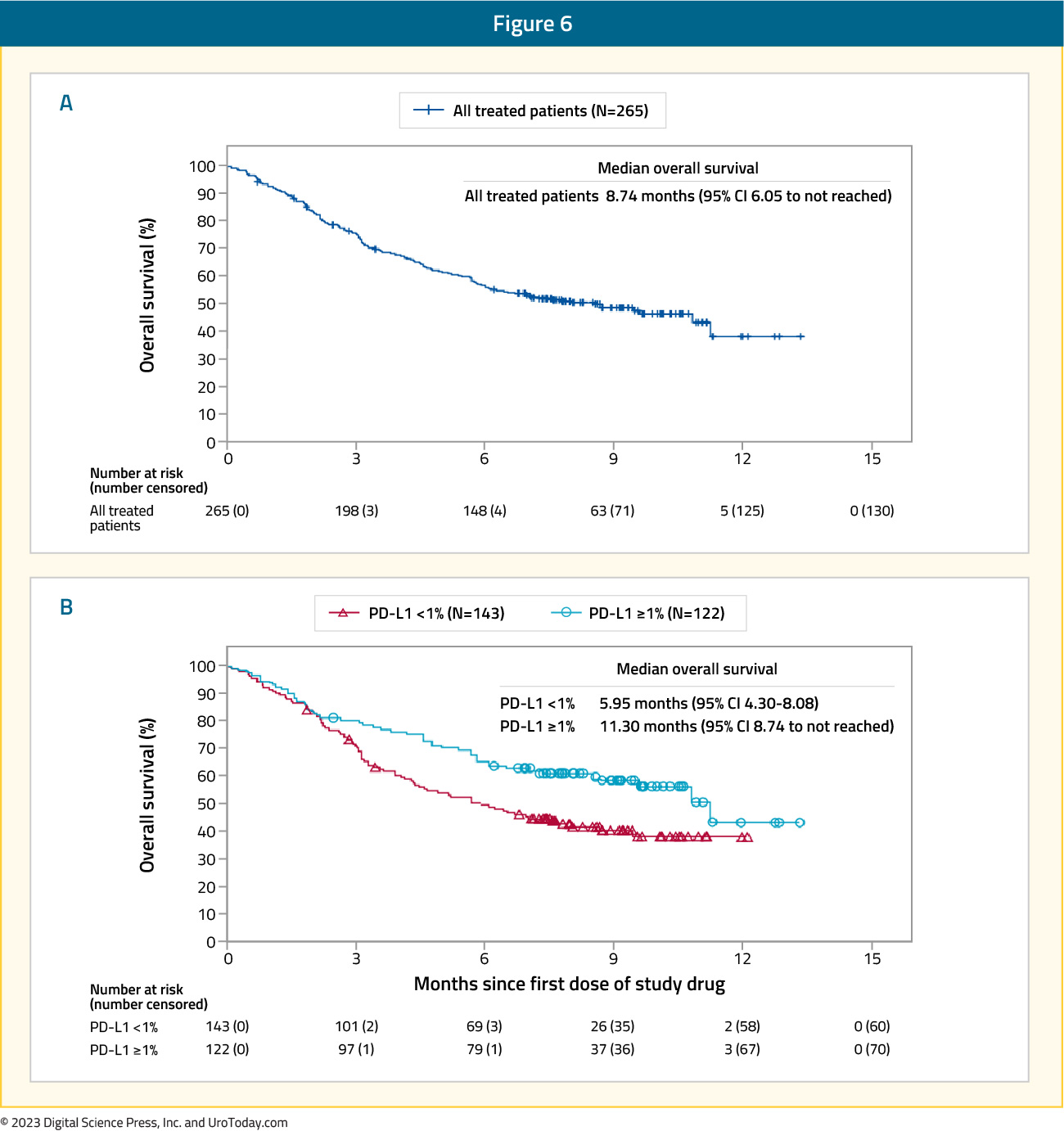
Based on these results from CheckMate 275, nivolumab monotherapy is an option for the second line treatment of metastatic urothelial carcinoma patients following first-line platinum-based chemotherapy.
In 2017, Apolo and colleagues assessed the safety and antitumor activity of avelumab in a phase 1b trial among patients with refractory metastatic urothelial carcinoma.12 There were 54 patients included that were unselected for PD-L1 expression and received avelumab 10 mg/kg every two weeks, with the primary objective being safety and tolerability, and secondary objectives including objective response rate, progression-free survival, and overall survival. Over a median follow-up of 16.5 months, grade 3-4 adverse events occurred in 6.8% of patients including asthenia, AST elevation, and decreased appetite. The confirmed objective response rate was 18.2% (95% CI: 8.2 – 32.7), with five complete responses and three partial responses. The median progression free survival was 11.6 weeks (95% CI: 6.1 – 17.4) and median overall survival was 13.7 months (95% CI: 8.5 – NE):
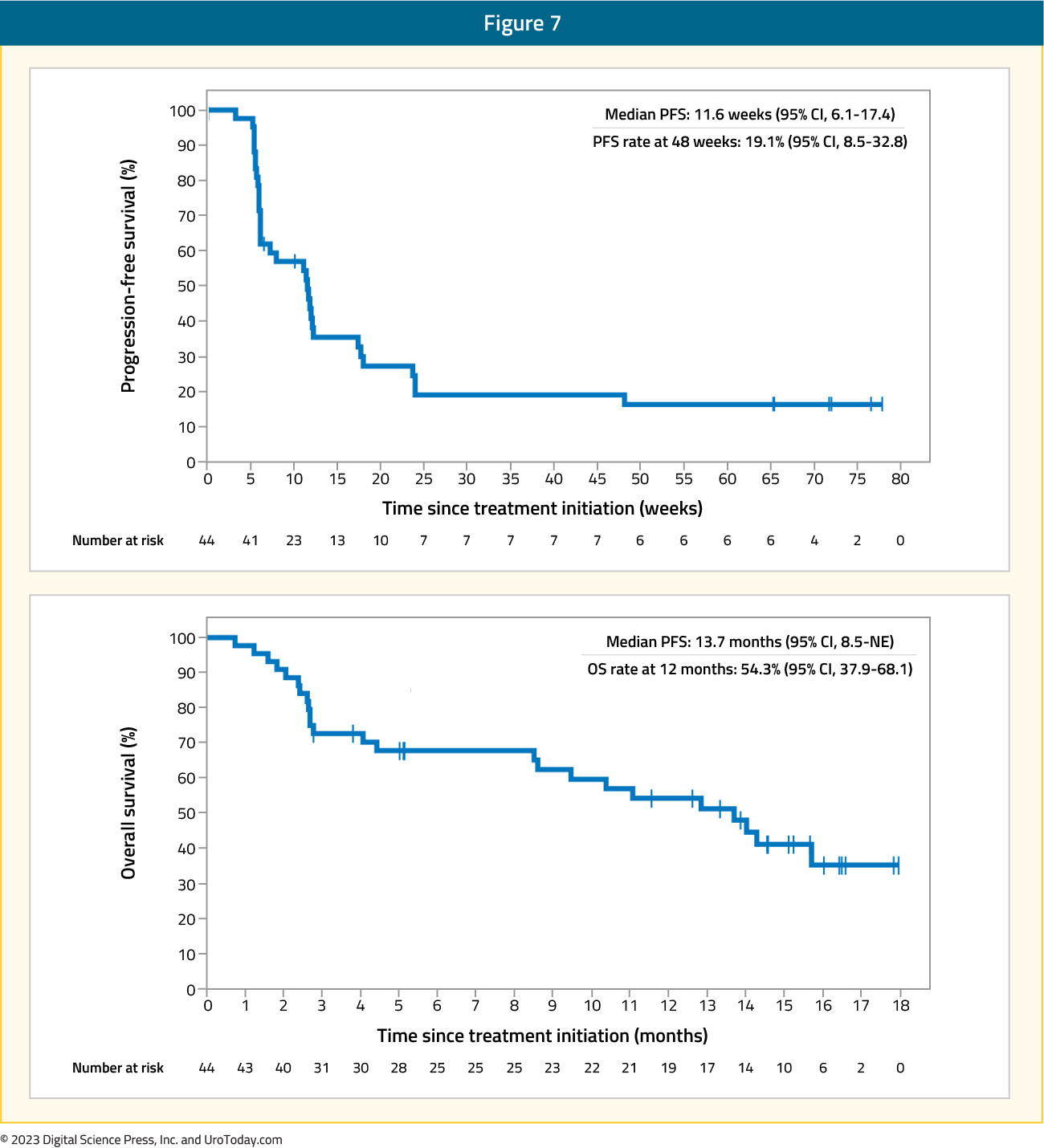
In 2019, results of the BLC2001 phase 2, open-label trial assessing erdafitinib in locally advanced and metastatic urothelial carcinoma were published in the New England Journal of Medicine.13 Erdafitinib is a potent tyrosine kinase inhibitor of fibroblast growth factor receptor 1-4 (FGFR1-4), which is a new, significant treatment given that as many as 20% of patients with advanced urothelial carcinoma have FGFR alterations.14 All patients included in this trial had prespecified FGFR alterations and a history of disease progression during or after at least one course of chemotherapy or within 12 months of neoadjuvant or adjuvant chemotherapy. The trial design for BLC2001 is as follows:

The primary endpoint was objective response rate, which was 40%, including 3% of patients having a complete response and 37% having a partial response. The median progression free survival was 5.5 months (95%: CI 4.2 – 6.0):
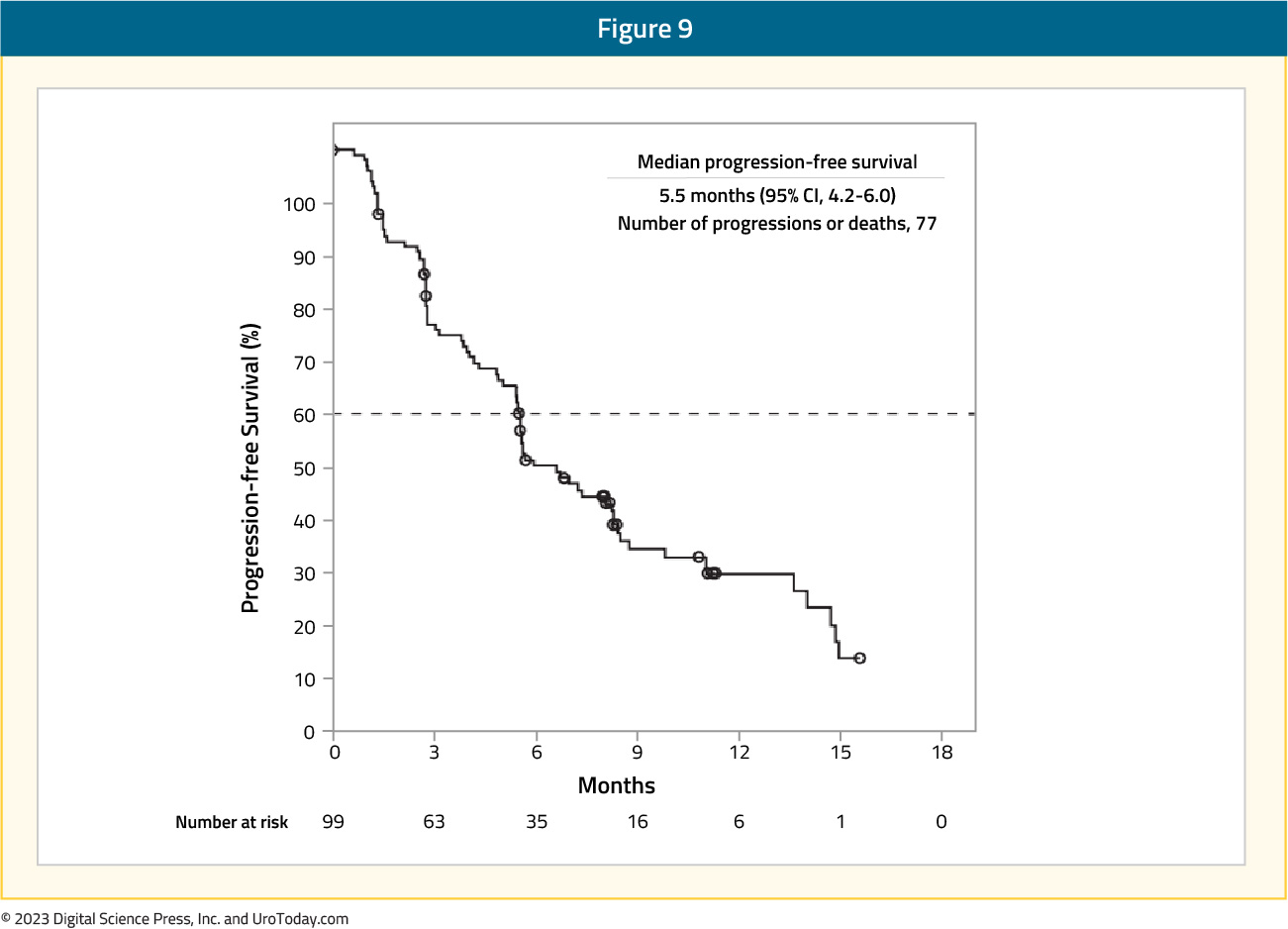
and median overall survival was 13.8 months (95% CI: 9.8 – not reached):
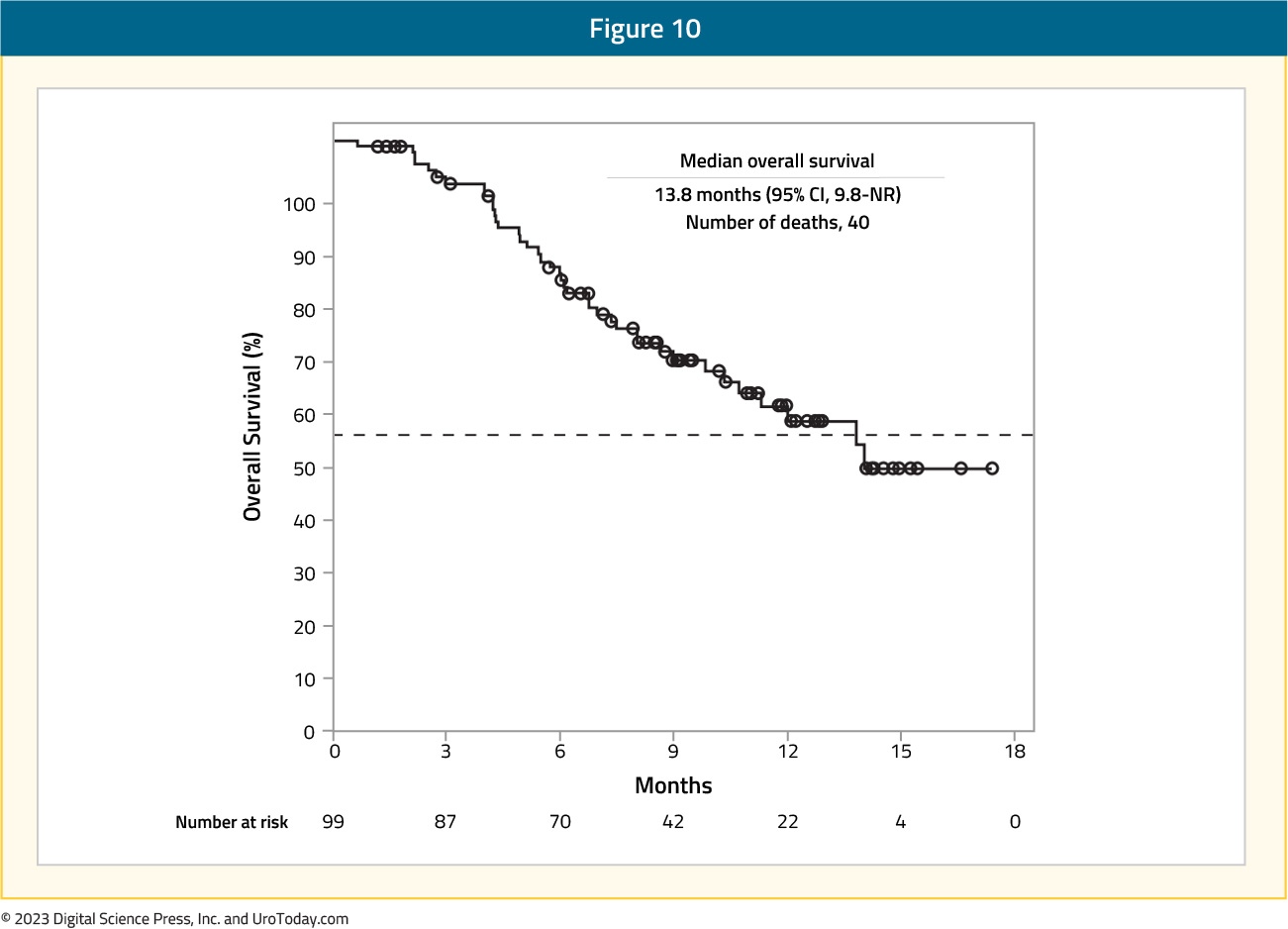
There were adverse events of grade ≥3 that occurred in 46% of patients, most commonly hyponatremia (11%), stomatitis (10%), and asthenia (7%).
At GU ASCO 2023, Dr. Petros Grivas and colleagues presented updated results of the single arm TROPHY-U-01 Cohort 3 trial, which included patients that had progression of metastatic urothelial carcinoma following platinum-based chemotherapy in the metastatic setting or ≤12 months following neoadjuvant/adjuvant platinum in the curative intent setting. Importantly, no prior checkpoint inhibitor therapy was allowed. The treatment regimen included 10 mg/kg of sacituzumab govitecan on day 1 and day 8 + 200 mg of pembrolizumab on day 1 of a 21-day cycle for up to two years. The primary endpoint was objective response rate per central review by RECIST 1.1. Among 41 treated patients and over a median follow-up of 12.5 months (range: 0.9 – 24.6), the objective response rate was 41%. This included a 20% complete response rate and 46% clinical benefit rate. Among evaluable patients, 82% experienced a reduction in target size lesion:
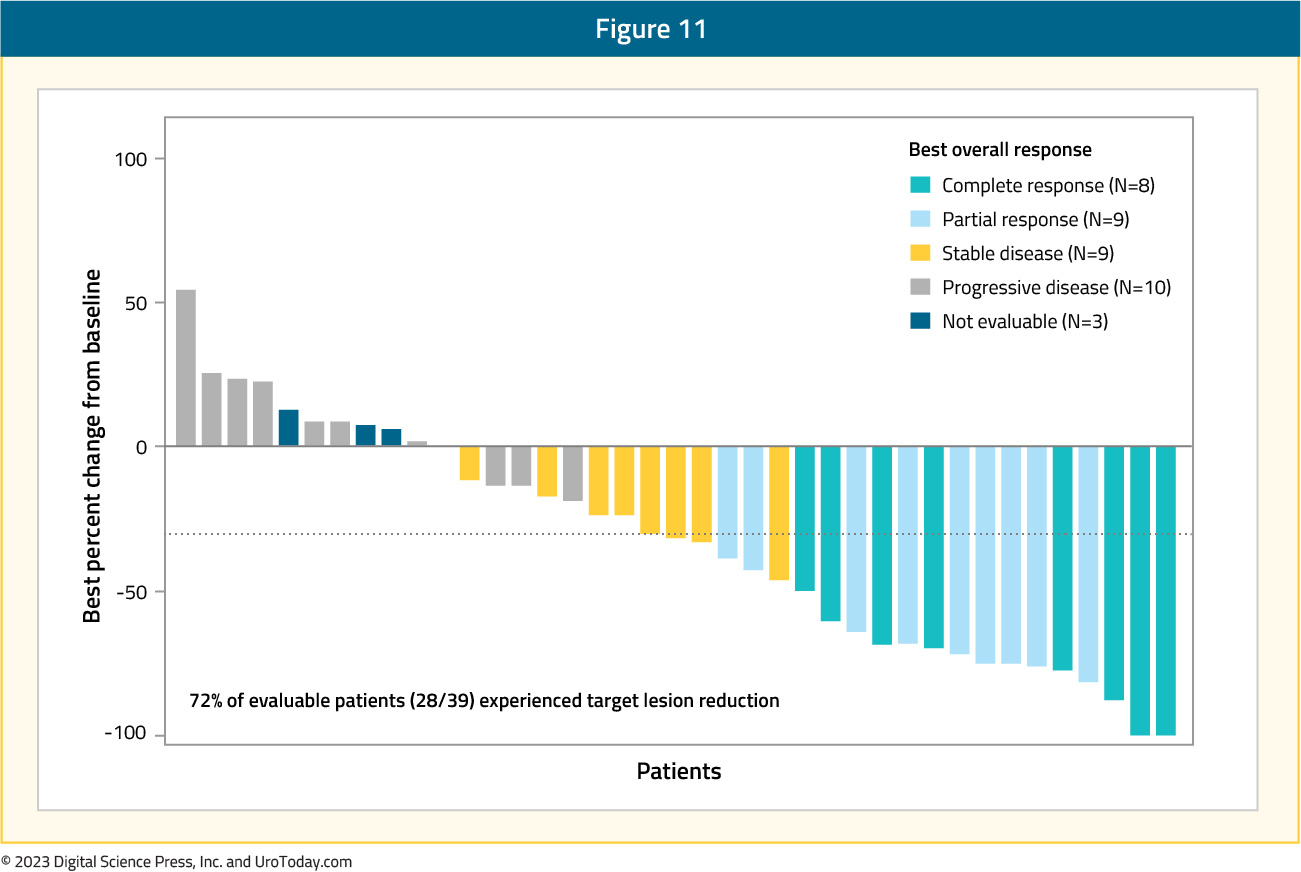
The median duration of response was 11.1 months (95% CI: 4.8 - NE), median progression free survival was 5.3 months (95% CI: 3.4 - 10.2), median time to response was 1.4 months (95% CI: 1.3 - 2.7), and median overall survival was 12.7 months (95% CI: 10.7-NE):

Other, primarily historical, agents that may be considered in select circumstances for second-line therapy among those progressing after platinum based chemotherapy include paclitaxel or docetaxel. Sideris and colleagues assessed the efficacy of weekly paclitaxel as a single agent chemotherapy following first-line cisplatin chemotherapy.15 Among 42 patients, all received weekly paclitaxel (80 mg/m2) with a median duration of 3 months. The overall response rate was 9.5%, disease control rate was 45.2%, and median progression free survival was 6.4 months. In this cohort, grade 3/4 toxicities were rare, including anemia (7%) and fatigue (2%), although 19% of patients had a pulmonary embolism. McCaffrey and colleagues8 have previously assessed the use of docetaxel in a phase 2 trial of 30 patients for second-line therapy. Among this cohort, four patients (13.3%) demonstrated a partial response, with duration of response ranging from 3 to 8 months. The median overall survival among all patients was 9 months (95% CI 6 – 12), with dose reductions secondary to adverse events necessary for 60% of patients.
Second Line Systemic Therapy after Post-Checkpoint Inhibitor
Among patients who received checkpoint inhibitor therapy in the first-line setting, the preferred second-line therapy is stratified by cisplatin eligibility. For those that are cisplatin eligible, patients should be considered for gemcitabine + cisplatin16 or dose dense MVAC,1 as discussed in the Center of Excellence article highlighting first line systemic therapy for cisplatin eligible patients. For those in the second line setting that are cisplatin ineligible, one of the preferred options is gemcitabine + carboplatin, as discussed in the Center of Excellence article highlighting first line systemic therapy for cisplatin ineligible patients.3 Additionally, patients may be considered for enfortumab vedotin based on data from the EV-201 trial.17 This trial was a multicenter, single arm, phase 2 study assessing enfortumab vedotin in patients with locally advanced or metastatic urothelial carcinoma who had previously been treated with PD-1 or PD-L1 inhibitors. These patients were deemed cisplatin ineligible and subsequently treated with intravenous enfortumab vedotin (1.25 mg/kg) on days 1, 8, and 15 of every 28-day cycle. Among 91 patients who were enrolled globally, 89 patients received treatment. Over a median follow-up of 13.4 months, the confirmed objective response rate (primary outcome) was 52% (95% CI: 41 – 62), with 20% of patients achieving a complete response and 31% a partial response:

The median progression free survival in EV-201 was 5.8 months, with an estimated progression-free survival at 6 months of 50% and 33% at 12 months:
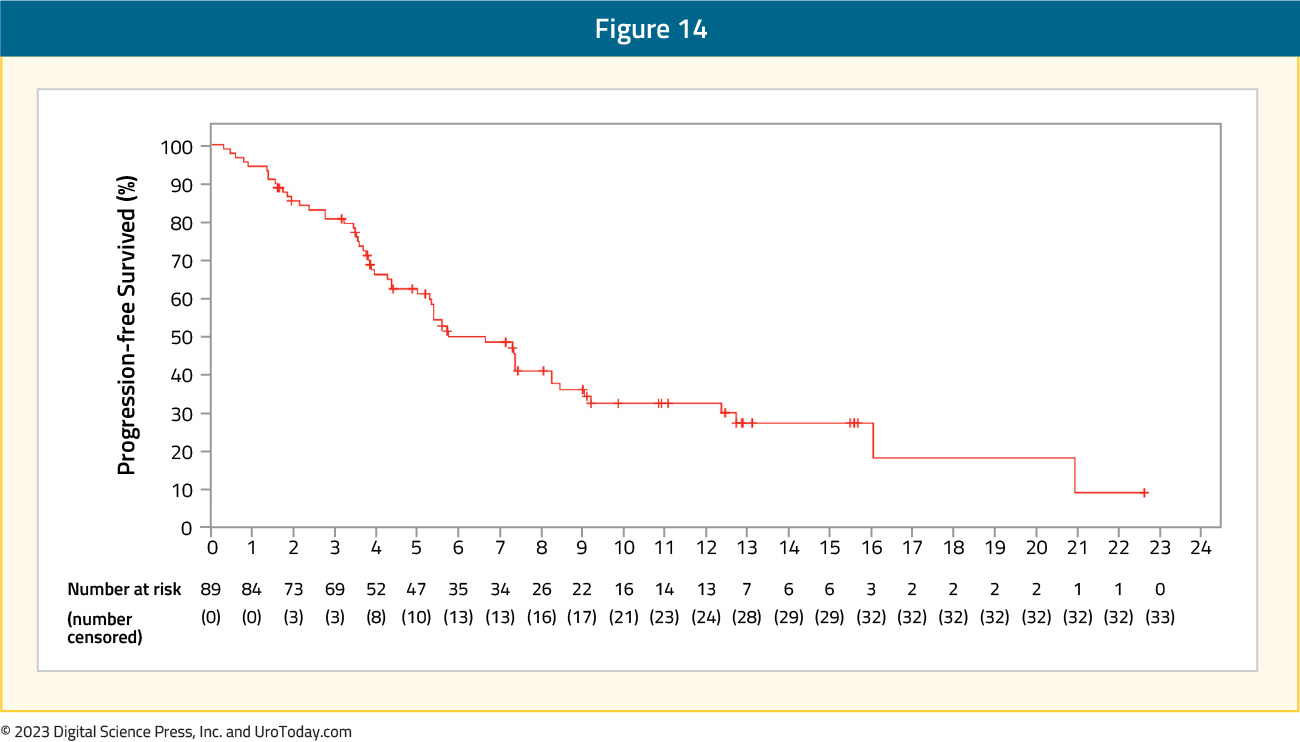
The median overall survival was 14.7 months; the most common grade 3 or 4 adverse events were neutropenia (9%), maculopapular rash (8%), and fatigue (7%). Given that this population of metastatic bladder cancer patients has few treatment options and secondary to the objective response rate of 52%, enfortumab vedotin is a promising new therapy in this disease space.
Similar to second line therapy patients who received platinum-based chemotherapy in the first line setting, patients who received checkpoint inhibitors in the first line may also be considered for docetaxel,8 paclitaxel,15 or erdafitinib.13
Conclusions
Until recently, there were very few treatment options available for patients with metastatic urothelial carcinoma in the second line setting. In addition to the utility of pembrolizumab as second-line therapy, recent advances in tumor molecular profiling have provided information regarding the prevalence of FGFR mutations and led to the development and approval of erdafitinib in these select patients. Continued efforts to delineate the tumor genetic profile and microenvironment will hopefully continue to provide additional mechanisms for which to target new therapies for patients with metastatic urothelial carcinoma after first line therapies.Published September 2023


