Since the beginning of the COVID-19 pandemic in early 2020, the diagnosis, treatment and surveillance of cancer has been transformed globally. The heavy demand for resources, exacerbated by limited excess health system capacity, means that health care systems have become quickly overwhelmed and hospitals have become sources for virus transmission.
Furthermore, a severe COVID-19 phenotype is seen more commonly in men and older, more comorbid patients.1 Indeed, this is the same comorbidity profile common for many patients with genitourinary malignancies. The need for repeated in-person interactions has hampered the ongoing accrual and conduct of prospective research during the COVID-19 pandemic. In many regions, accrual to prospective studies has entirely stopped as a result of the pandemic. In jurisdictions which are particularly hard hit, many research personnel and research-related resources have been re-directed and re-deployed into both COVID-related clinical care and COVID-related research.2
Clinical trials represent a key platform of comprehensive cancer centers and may provide unique therapeutic options in patients with aggressive malignancies with limited standard therapies. Additionally, previous studies have suggested that patients enrolled in clinical trials may have improved outcomes3 and may receive more conscientious nursing care,4 thus further highlighting the importance of continuing a clinical trial platform even during difficult times. The coronavirus has the potential to impact the integrity and patient safety of ongoing trials as well as increase the operational burden on trial programs, therefore potentially limiting access to trials and new therapies for oncology patients.5 Opportunities for clinical trial enrollment may still be provided to patients during the COVID-19 outbreak, but likely require thorough evaluation on a case-by-case basis.6
As clinicians, it is important to be good stewards of resources, patient safety, and community health initiatives. But at the same time, we must prioritize oncology patients for treatment and continue exploring new therapeutic options for patients through accrual to clinical trials. In the absence of data, the guidance of care and the landscape of clinical trials relies on expert opinion, including a collaborative review in the press in European Urology by Wallis et al.7 This article will discuss clinical trial challenges secondary to the COVID-19 pandemic, provide guidance from governing bodies for clinical trial conduct, and discuss the future implications of clinical trials following the COVID-19 pandemic.
Clinical Trial Challenges During COVID-19
During the COVID-19 pandemic, several challenges have emerged with regards to conducting clinical trials in the traditional manner we have used for decades. Clinical trialists at pan-Asian academic centers running non-industry sponsored multi-center trials have noted several concerns.8 First, there has been difficulty systematically gathering information regarding restrictions at each site and how these restrictions affect trial conduct. Second, there are no clear guidelines for trial management committees on their ethical obligations under these circumstances. Furthermore, there is no guidance for investigators or trial participants. Third, despite public safety being paramount, the impact on clinical trials is potentially enormous, so that rational measures to prevent a complete collapse of the clinical trial infrastructure is vital. Fourth, recruitment may be reduced or halted, perhaps for a prolonged period, thus extending trial timelines and depleting limited budgets. Costs will persist even during trial inactivity.Additional considerations include the logistic consequences of disrupting drug supplies and increased loss to follow-up because of lockdown, travel restrictions, or patients avoiding hospitals secondary to COVID-19 infection concerns.8 Statistical considerations include the impact of treatment disruptions on endpoints, including potential alteration of event rate, including death from all causes, higher rates of missing data, and delay in routine surveillance of endpoints, which could falsely increase disease-free survival rates. Clinical trial staffing may be affected by illness and quarantine, as well as the diversion of duties. Governing and regulatory bodies may need to cancel or delay site visits, providing further delays in protocol approvals and participant recruitment. Finally, biospecimen collection protocols may be affected, including throat swab and saliva collections being discouraged given the COVID-19 infectious risk.
Guidance for Clinical Trial Conduct
There are several important points to consider regarding the feasibility of clinical trial enrollment during the COVID-19 pandemic. First, considerations should be made as to whether clinical investigators and supporting staff are able to safely comply with the trial requirements and guarantee patients’ compliance. Second, a patient’s ability and risk to travel for therapy during a time of rigorous social distancing and household quarantine must be considered. Third, in an attempt to offer optimal therapeutic options with inclusion in clinical trials, investigators should carefully evaluate the risks of adding extra-delays in treatment initiation due to administrative issues or bureaucratic constraints related to the COVID-19 pandemic and extra visits requiring travel associated with a higher risk of infection in this largely vulnerable population. Early evidence from China has suggested that investigators who continue to enroll in clinical trials during the COVID-19 pandemic should expect frequent protocol violations, with an average deviation of 27 days +/- 13 days.9In March 2020, the American Society of Clinical Oncology (ASCO) launched a survey of clinical programs represented in the Cancer Research Committee and Research Community Forum Steering Group, as well as taskforces to learn about the changes and challenges that clinical trial programs were experiencing early in the pandemic.10 Respondents indicated that COVID-19 is, unfortunately, leading programs to halt or prioritize screening and/or enrollment for certain clinical trials and completely eliminate research-only visits. Numerous challenges with conducting clinical trials were reported, including enrollment and protocol adherence difficulties with decreased patient visits, staffing constraints, and limited availability of ancillary services.
The following strategies for conducting clinical trials during the COVID-19 pandemic were provided from the ASCO survey:10
- Keep clinical trial participants informed about changes to clinical trials and remind participants to contact the research team with health changes
- Use the COVID-19 pandemic to develop standard operating procedures for clinical trials that may be used in the setting of future coronavirus waves of infection and other disease outbreaks
- Utilize e-signatures for informed consent and other trial-related documents
- Organize frequent staff meetings to discuss clinical trial operational challenges and provide trial updates
- More frequent utilization of telehealth visits for patients rather than required in-person meetings
- Further leverage telehealth for documenting review of symptoms and adverse events while on trial
- Establish a system for prioritizing clinical trial resource allocation, whereby determining which trials should continue/maintain screening and enrollment
- Mandate remote study visits and monitoring from trial sponsors and contract research organizations
- Use remote safety lab collections when feasible and ship oral drugs directly to patients
- Communicate to clinical trial teams any changes or concerns about existing trials to the institutional review board committees
- Ensure thorough documentation of changes to procedures and modifications to or deviations from protocols, utilizing a “COVID-19” tag to facilitate ease of searching after the pandemic
- Ensuring the safety of trial participants is paramount. Study decisions may include those regarding continuing trial recruitment, continuing use of the investigational product for patients already participating in the trial, and the need to change patient monitoring during the trial
- Sponsors, in consultation with clinical investigators and review and ethics committees, may determine that the protection of a participant’s safety is best served by continuing a participant in the trial, or by discontinuing the administration of the investigational product or even participation in the trial
- Since trial participants may not be able to come to the site for protocol-specified visits, sponsors should evaluate whether alternative methods for safety assessments (ie. telehealth platforms) could be implemented, and would be sufficient to assure the safety of trial participants
- COVID-19 screening procedures that may be mandated by the clinical trial’s health care system do not need to be reported as an amendment to the protocol unless the sponsor is incorporating the data collected as part of a new research objective
- Sponsors and clinical investigators are encouraged to engage with review and ethics committees as early as possible when urgent or emergent changes to the protocol or informed consent are anticipated as a result of COVID-19
- Changes in study visit schedules, missed visits, or patient discontinuations may lead to missing information, and it will be important to capture specific information in the case report form that explains the basis of the missing data, including the relationship to COVID-19
- If changes in the protocol will lead to amending data management and/or statistical analysis plans, the sponsor should consider doing so in consultation with the applicable FDA review division
- If planned on-site monitoring visits are no longer possible, sponsors should consider optimizing the use of central and remote monitoring programs to maintain oversight of clinical sites
Future Implications for Clinical Trials
The COVID-19 pandemic has forced clinical trialists to re-evaluate how we conduct oncology clinical trials moving forward. One such platform that has been discussed is the ‘decentralization’ of clinical trials. This concept entails data being collected at remote locations, with the caveat that data collection may also be virtual.12 This is in contrast to traditional clinical trials where data must be collected at the designated research facility or via contract research organizations. Decentralized trials do not compromise the study design or statistical consideration but only refers to the locality and method of data collection.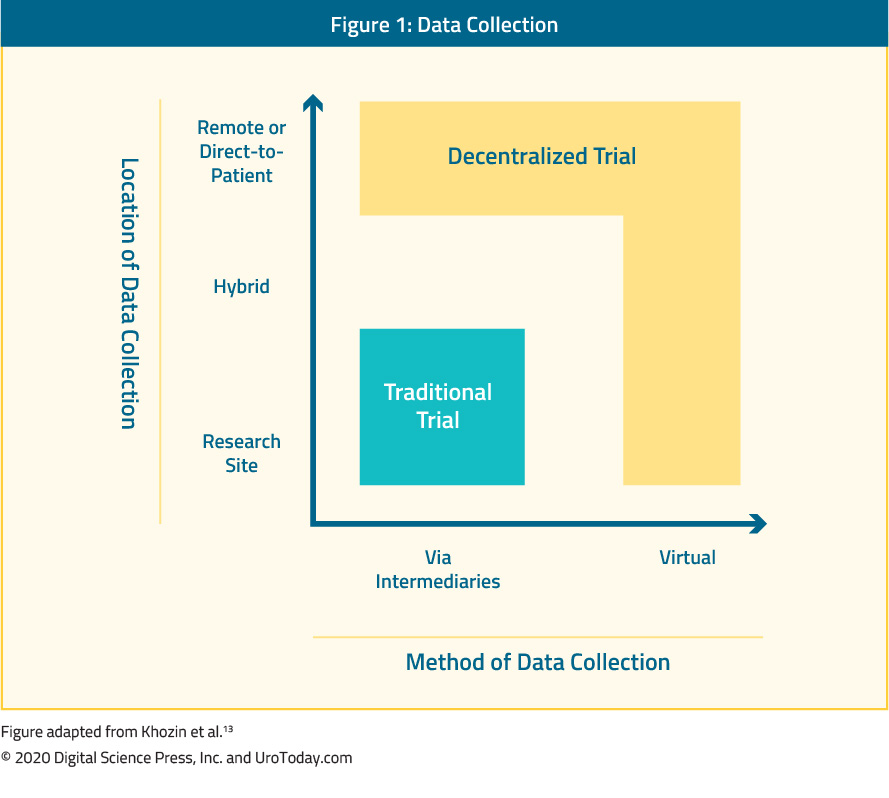
Several potential barriers to decentralized clinical trial models have been identified including: (i) a potentially greater reliance on data security,13 (ii) additional training, insurance and indemnity considerations, and (iii) management of serious adverse events. Furthermore, oral therapies are likely more suited for a decentralized platform compared to parenteral therapies. Early phase trials (ie. phase I trials) with intense and specialized pharmacokinetic and pharmacodynamic sample collection requirements may not be well suited for decentralized data collection. The key to decentralized clinical trials is maintaining the quality and integrity of data, which can be collected remotely and away from the primary study site.
Telehealth platforms, as has been previously discussed, have transformed clinical care during the COVID-19 pandemic and may also be incorporated into clinical trials. While not suitable for all trials or interventions, Galsky and colleagues demonstrated the feasibility of a telemedicine-based interventional oncology trial.14 Following a single, in-person enrollment visit, the investigators utilized telehealth interactions using a smartphone-based platform for ongoing follow-up in their trial of metformin in patients with biochemically recurrent prostate cancer. Indeed, the level of safety monitoring that is considered “good practice” may be unnecessary for certain investigational therapeutics or a subset of low-risk clinical trial participants.15,16 Uren et al.17 observed that remote monitoring for complex Phase III clinical trials was feasible and led to an overall cost saving for the sponsor and reduced burden on facility resources. Furthermore, reducing the intensity of in-person visits for cancer clinical trials may help promote equity in recruitment and improved access. Certainly, how telehealth is incorporated into clinical trials moving forward will undoubtedly continue to evolve.
Conclusions
Clinical trials are fundamental for medical progress, but their vulnerability to external forces is highlighted by the recent coronavirus outbreak. The global clinical trial community has been hampered with a lack of contingency plans. A rational, carefully adapted policy encompassing operational responses to such crises should be a priority moving forward. Reevaluating the dated model of repeated in-person visits should be encouraged, capitalizing on major advances in telehealth platforms and the decentralization of clinical trials.Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Atlanta, Georgia
Published Date: May 2020


