Why is this trial important?
Darolutamide is a structurally distinct androgen receptor pathway inhibitor (ARPI), with low blood-brain penetration and decreased drug-drug interactions. Darolutamide has been previously approved for mHSPC in combination with docetaxel and ADT based on the results of the phase III ARASENS trial.1 In ARASENS, darolutamide + ADT + docetaxel significantly improved median overall survival (OS; not reached)) versus ADT + docetaxel (median 48.9 months; HR 0.68, 95% CI 0.57–0.80) in patients with mHSPC.
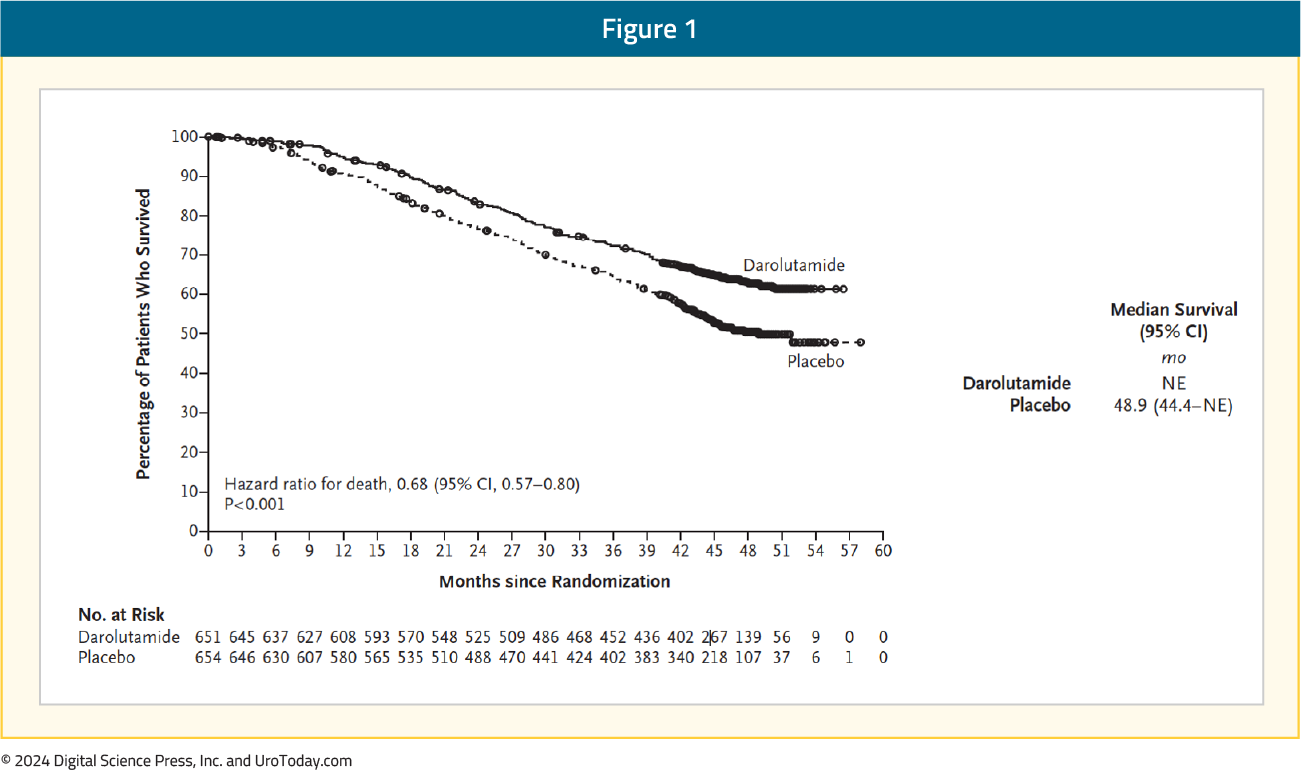
Furthermore, the incidence of treatment-emergent adverse events (TEAEs) was similar in both groups and consistent with a chemotherapy-treated patient cohort. Because of the tolerability of darolutamide, in addition to some practitioners and patients being reticent to prescribing/receiving chemotherapy, evaluating the role of darolutamide + ADT without docetaxel may provide new treatment options for patients with mHSPC.
What is the trial design?
ARANOTE is a phase III trial of mHSPC by conventional imaging, randomizing patients 2:1 to darolutamide 600 mg twice daily + ADT versus placebo + ADT. Eligible patients had mHSPC, ECOG performance status 0–2, and were stratified based on the presence of visceral metastases (Y/N) and prior local therapy (Y/N). The primary endpoint was radiological progression-free survival (rPFS), and secondary endpoints included OS, time to initiation of subsequent anticancer therapy, time to CRPC, time to PSA progression, time to pain progression, and safety:
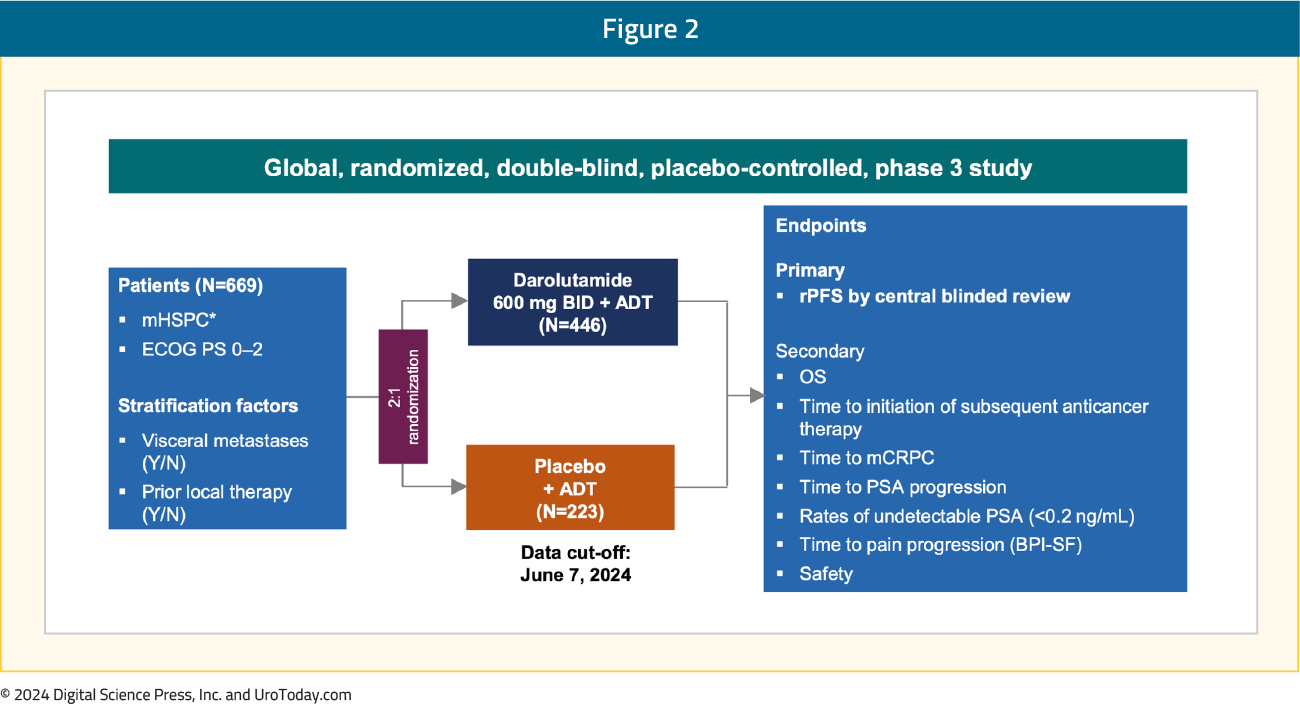
What were the key findings?
Darolutamide + ADT significantly improved rPFS versus placebo + ADT (HR 0.54, 95% CI 0.41–0.71):
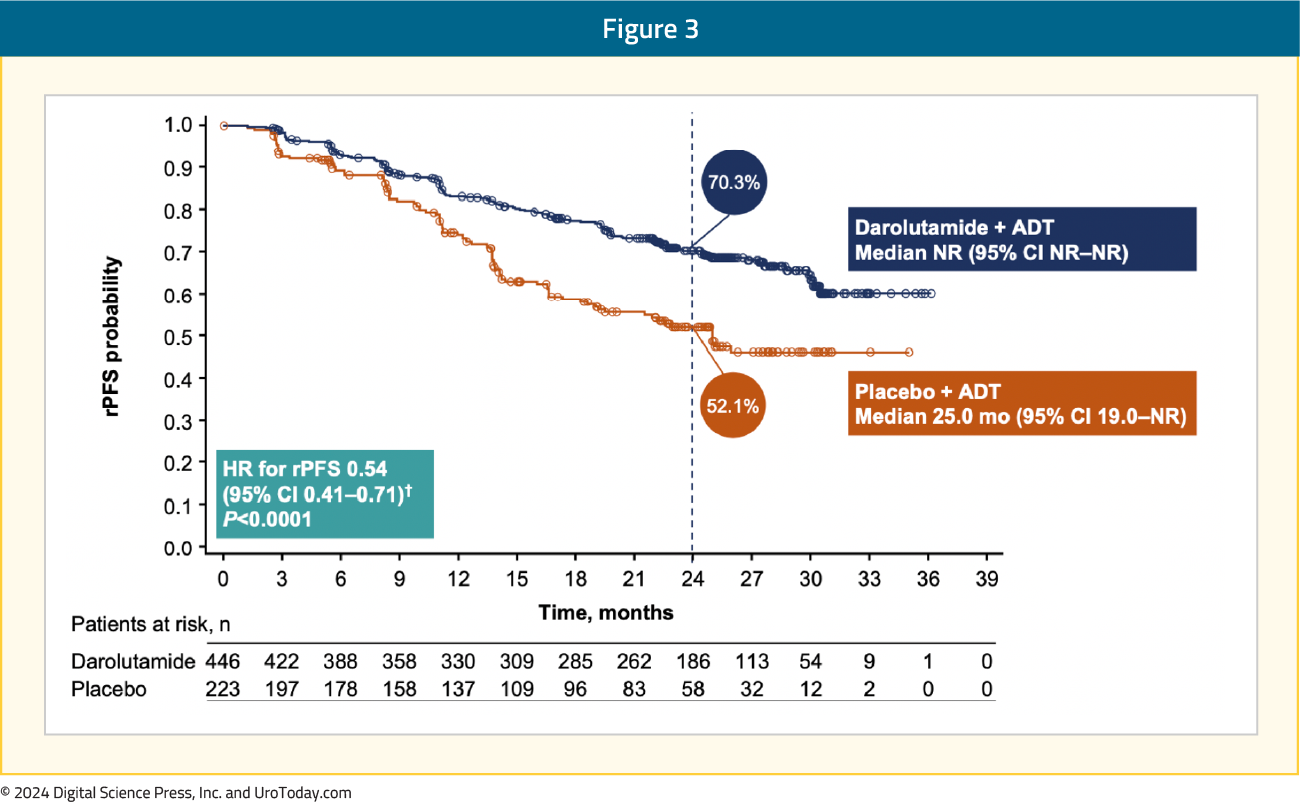
The rPFS benefit was consistent across subgroups, in particular for patients with low volume mHSPC:
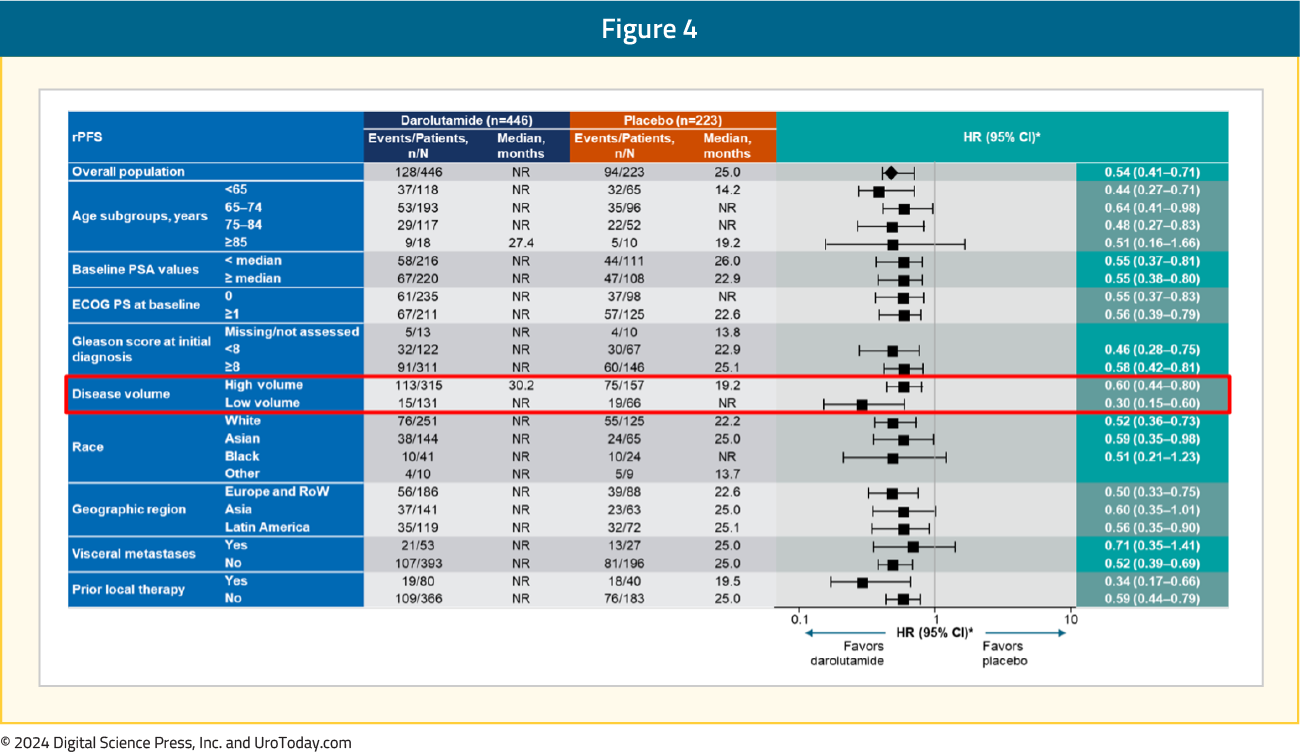
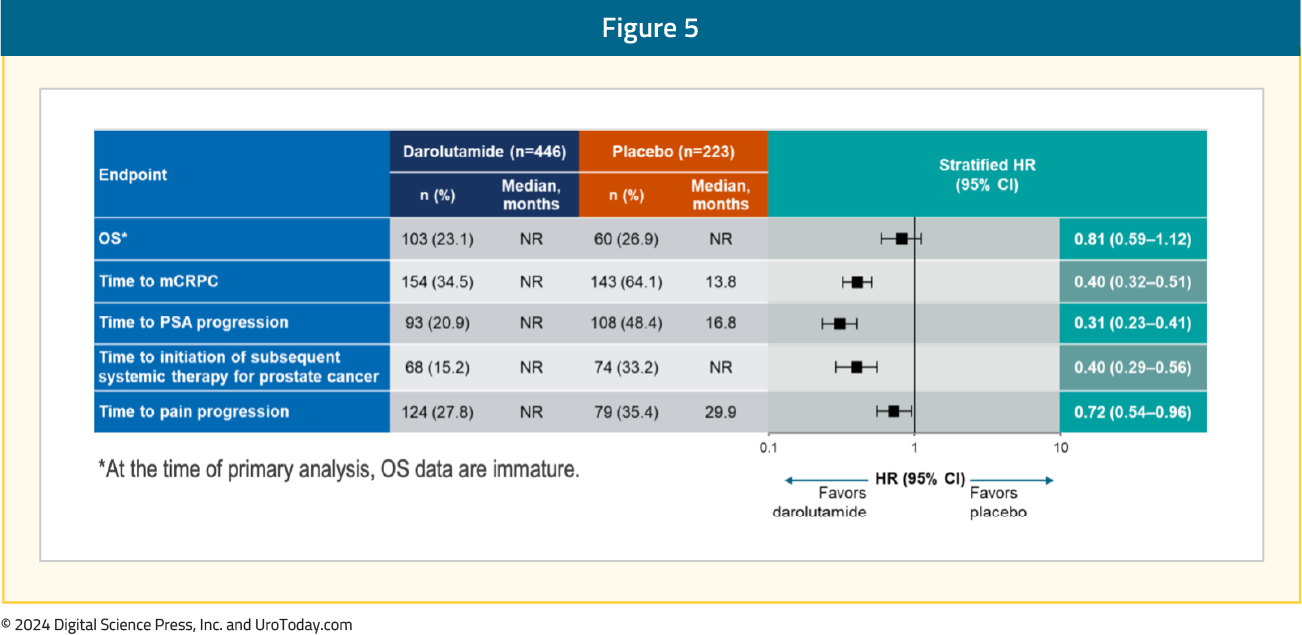
Moreover, darolutamide was associated with a positive trend for OS (HR 0.81, 95% CI 0.59–1.12) and clinical benefits across all secondary efficacy endpoints, including time to CRPC (HR 0.40, 95% CI 0.32–0.51), time to PSA progression (HR 0.31, 95% CI 0.23–0.41), time to subsequent therapy (HR 0.40, 95% CI 0.29–0.56), and time to pain progression (HR 0.72, 95% CI 0.54–0.96):
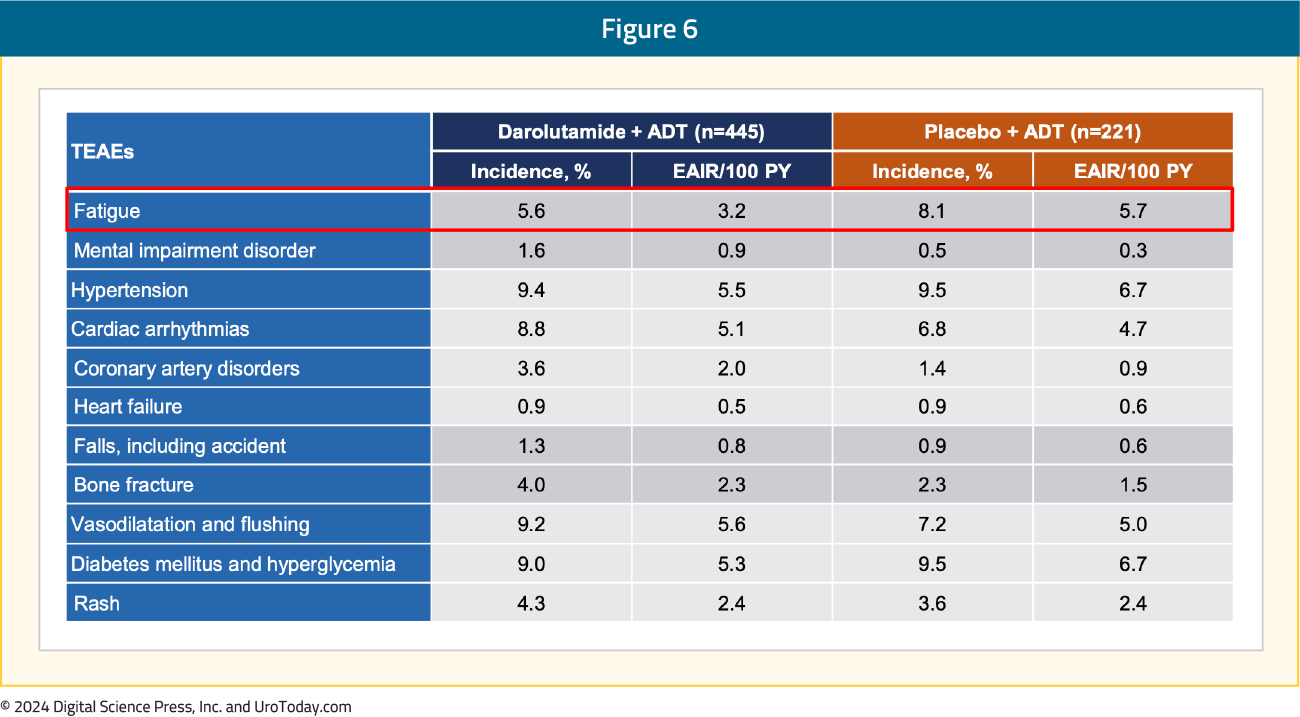
With regards to TEAEs of special interest, events were comparable between the two arms. Notably, there was less fatigue (5.6%) in the darolutamide + ADT arm compared to the placebo + ADT arm (8.1%):
What are the clinical implications?
These results from the second pivotal trial of darolutamide in patients with mHSPC add to the body of evidence from ARASENS and provide the option to tailor treatment intensification in mHSPC, with or without docetaxel, to meet a patient’s individual needs and preferences. This clinical trial population may more accurately represent the ‘real-world’ population we treat in clinical practice – this was a highly diversified population, including elderly patients, 31% Asian, 10% Black, and 29% accrued from Latin America. In an elderly population, no increase in the incidence of fatigue for darolutamide + ADT (in fact, numerically less than ADT alone) is noteworthy when discussing clinical efficacy and quality of life with patients. Although this study was powered on rPFS (and not OS), the efficacy of darolutamide in ARANOTE, including the secondary outcome of OS, are consistent with the findings from the ARCHES trial2 of enzalutamide + ADT. Particularly among patients with low volume mHSPC (HR 0.30, 95% CI 0.15-0.60), darolutamide + ADT may be a reasonable treatment option.
Published October 2024

