Library Resources
- Written by: Rashid Sayyid MD, MSc and Zachary Klaassen, MD, MSc
- References:
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369(3):213-223.
- Parsons MW, Tward JD. Utilization of Radium-223 in Metastatic Prostate Cancer Patients and Novel Prognostic Indicators in Radium-223 Patients. Int J Rad Oncol Biol Phys. 2020;108(3):e899.
- Ahmadzadehfar H, Azgomi K, Hauser S, et al. 68Ga-PSMA-11 PET as a Gatekeeper for the treatment of metastatic prostate cancer with 223Ra: Proof of Concept. J Nucl Med. 2017; 58(3):438-444.
- Rahbar K, Essler M, Eiber M, et al. Safety and Effectiveness of Lutetium-177-Prostate Specific Membrane Antigen (177Lu-PSMA) Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC) Previously Treated with Radium-223 (223Ra): The RALU Study. J Nucl Med. 2023; 64(12):1925-1931.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368(2):138-148.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014; 371(5):424-433.
- Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019; 20(3):408-419.
- Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med. 2023; 389(16):1453-1465.
- Leith A, Ribbands A, Kim J, et al. Impact of next-generation hormonal agents on treatment patterns among patients with metastatic hormone-sensitive prostate cancer: a real-world study from the United States, five European countries and Japan. BMC Urol. 2022; 22(1):33.
International Bladder Cancer Group (IBCG) at the American Urological Association (AUA) 2024
- Written by: Bogdana Schmidt, MD, MPH, Assistant Professor Urologic Oncology, University of Utah, Huntsman Cancer Institute, Salt Lake City, Utah
ESMO 2024 Quick Take Insights: Prostate Cancer
Introduction
At the ESMO 2024 annual meeting in Barcelona, Spain, the results of numerous practice-changing trials were presented, including those from three highly-anticipated clinical trials in the advanced prostate cancer space:
- Written by: Zachary Klaassen, MD, MSc, Wellstar MCG Health, Augusta, GA and Rashid Sayyid, MD, MSc, University of Southern California, Los Angeles, CA
- References:
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022; 386(12):1132-1142.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368(2):138-148.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014; 371(5):424-433.
- Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019; 20(3):408-419.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021; 385(12):1091-1103.
European Association of Urology (EAU) 2024: Bladder Cancer Highlights
- Written by: Amanda Myers, MD, Fellow of Urologic Oncology, MD Anderson Cancer Center, Houston, Texas
ESMO 2024 Quick Take Insights: A Focus on PEACE-3
ESMO 2024 Quick Take Insights: A Focus on PEACE-3
The ESMO 2024 annual meeting in Barcelona, Spain, featured many practice-changing trials and hypothesis-generating data in genitourinary cancer. One of the most highly anticipated trials in the metastatic castration-resistant prostate cancer (mCRPC) disease space was the phase III PEACE-3 trial – the focus of this ESMO 2024 Quick Take Insights.
- Written by: Zachary Klaassen, MD, MSc Wellstar MCG Health, Augusta, GA & Rashid Sayyid, MD, MSc, University of Southern California, Los Angeles, CA
- References:
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368(2):138-148.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014; 371(5):424-433.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Rahbar K, Essler M, Pabst KM, et al. Safety and Survival Outcomes of 177Lu-Prostate-Specific Membrane Antigen Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer with Prior 223Ra Treatment: The RALU Study. J Nucl Med. 2023 Apr;64(4):574-578.
- Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019; 20(3):408-419.
American Urological Association (AUA) 2024 Annual Meeting Summary
- Written by: Patrick J. Hensley, MD, Urologic Oncologist, University of Kentucky College of Medicine Lexington, KY, USA
ESMO 2024 Quick Take Insights: A Focus on ARANOTE
- Written by: Zachary Klaassen, MD, MSc Wellstar MCG Health, Augusta, GA & Rashid Sayyid, MD, MSc, University of Southern California, Los Angeles, CA
- References:
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022; 386(12):1132-1142.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019 Nov 10;37(32):2974-2986.
Indwelling Urinary Catheter Management of the Acute Patient: Quality Improvement Issue Brief
- Written by: Diane K. Newman DNP, ANP-BC, FAAN
ESMO 2024 Quick Take Insights: A Focus on SPLASH
ESMO 2024 Quick Take Insights: A Focus on SPLASH
The ESMO 2024 annual meeting in Barcelona, Spain, featured many practice-changing trials and hypothesis-generating data in genitourinary cancer. One of the most highly anticipated trials in the metastatic castration-resistant prostate cancer (mCRPC) disease space was the phase III SPLASH trial – the focus of this ESMO 2024 Quick Take Insights.
- Written by: Zachary Klaassen, MD, MSc Wellstar MCG Health, Augusta, GA & Rashid Sayyid, MD, MSc, University of Southern California, Los Angeles, CA
- References:
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021; 385(12):1091-1103.
- Morris MJ, Castellano D, Herrmann K, et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naïve patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): A phase 3, randomized, controlled trial. Lancet 2024 Sep 28;404(10459):1227-1239.
PARP Inhibitor Combination Therapy: Approved Treatments for Metastatic Castrate-Resistant Prostate Cancer Patients
Introduction
Given the promising results that Poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors have demonstrated for the treatment of metastatic castrate-resistant prostate cancer (mCRPC) patients who had progressed following prior androgen receptor pathway inhibitor (ARPI) and/or taxane-based chemotherapy, there has been increased interest in ‘moving up’ these agents along the disease spectrum, as well as combining them with other agents that may have a synergistic mechanism of action.- Written by: Zachary Klaassen, MD, MSc Associate Professor of Urology Urologic Oncologist Medical College of Georgia, Georgia Cancer Center Augusta, GA & Rashid Sayyid, MD, MSc Urologic Oncology Fellow University of Toronto Toronto, Ontario, Canada
- References:
- FDA Approves Olaparib with Abiraterone and Prednisone (Or Prednisolone) for BRCA-Mutated Metastatic Castration-Resistant Prostate Cancer.
- FDA Approves Niraparib and Abiraterone Acetate plus Prednisone for BRCA-Mutated Metastatic Castration-Resistant Prostate Cancer.
- FDA Approves Talazoparib with Enzalutamide for HRR Gene-Mutated Metastatic Castration-Resistant Prostate Cancer.
- Asim M, Tarish F, Zecchini HI, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun. 2017; 8:374.
- Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012; 2:1134-1149.
- Li L, Karanika S, Yang G, et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal. 2017;10: eaam7479.
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evidence. 2022;1(9).
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(10):1094-1108.
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2023;41(18):3339-3351.
- Chi KN, Sandhu S, Smith MR, et al. Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of the randomized phase III MAGNITUDE trial. Ann Oncol. 2023;34(9):772-782.
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023;402(10398):291-303.
- Fizazi K, Azad AA, Matsubara N, et al. First-line talazoparib with enzalutamide in HRR-deficient metastatic castration-resistant prostate cancer: the phase 3 TALAPRO-2 trial. Nat Med. 2024;30(1):257-264.
- Schaeffer EM, Srinivas S, Adra N, et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21(10):1067-1096.
- Kurian AW, Abrahamse P, Furgal A, et al. Germline Genetic Testing After Cancer Diagnosis. JAMA. 2023;330(1):43-51.
- Zhen JT, Syed J, Nguyen KA, et al. Genetic testing for hereditary prostate cancer: Current status and limitations. Cancer. 2018;124(15):3105-3117.
Metastasis-Directed Therapy in Oligometastatic Hormone-sensitive Prostate Cancer: New Frontiers in Advanced Prostate Cancer
Introduction
Classical theories of metastasis have followed ‘the seed and soil’ hypothesis, the Halstedian model, which proposes an orderly spread of disease from local to distant sites, with the presumption that cancer is an inherently systemic process even in the earliest cases. More contemporary spectrum theories now suggest that the propensity for distant spread exists along a ‘metastatic continuum’. Tumors with limited metastatic potential (i.e., oligometastases) represent a unique subset along this spectrum that could be potentially cured with local ablative therapy (i.e., metastasis-directed therapy [MDT]). This concept is not unique to prostate cancer and has been evaluated in other disease sites including non-small cell lung, colorectal, esophageal, and breast cancers.1
The majority of the evidence for MDT in the prostate cancer space, to date, has been for patients with recurrent, oligometastatic hormone sensitive prostate cancer. MDT has been evaluated both in lieu of, to avoid/delay the use of systemic therapy, and in combination with systemic therapy to potentially improve efficacy outcomes. In this Center of Excellence article, we discuss the evidence and practical applications for MDT in the recurrent oligometastatic hormone sensitive setting.
Trials of MDT versus Observation/Surveillance for Recurrent Oligometastatic Prostate Cancer
To date, three prospective phase II trials have compared MDT to observation for patients with recurrent oligometastatic hormone sensitive prostate cancer.
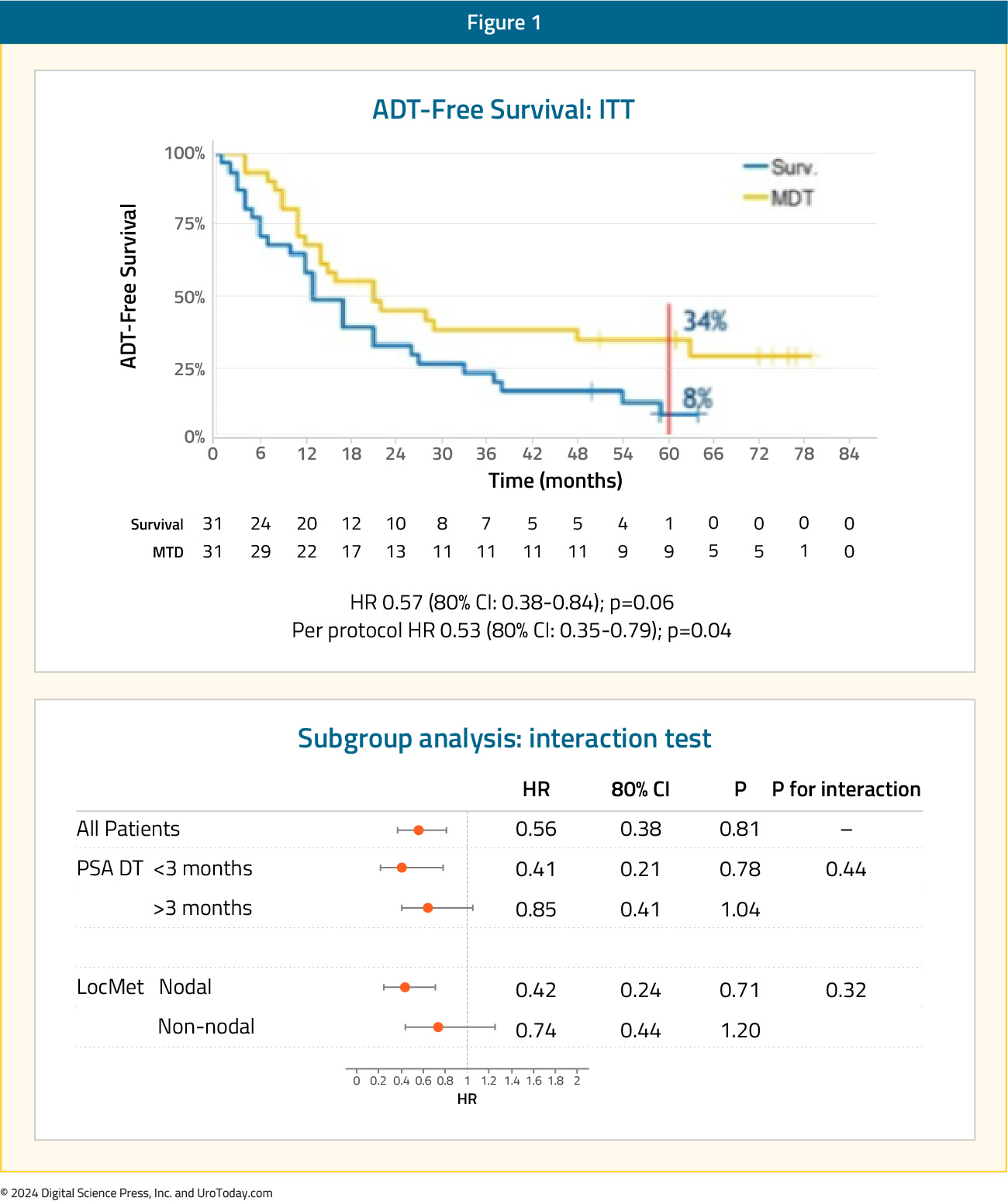
STOMPThe STOMP trial was a multicenter, randomized phase II trial that prospectively evaluated the effects of MDT for eugonadal men with evidence of oligometastatic disease on choline PET/CT (up to three extracranial sites) who had received prior treatment with curative intent and had evidence of biochemical recurrence. Between 2012 and 2015, 62 patients were randomized 1:1 to either surveillance or MDT, consisting of stereotactic body radiotherapy (SBRT) or metastasectomy. The primary endpoint was time to initiation of ADT (i.e., ADT-free survival). ADT was initiated for symptoms, progression beyond three metastases, or local progression of known metastatic disease
After a median follow up of 5.3 years, the five-year ADT-free survival was 8% in the surveillance arm compared to 34% for the MDT group (HR: 0.57, 95% CI: 0.38–0.84, log-rank p = 0.06). No differences were seen between groups when stratified by nodal versus non-nodal metastases:
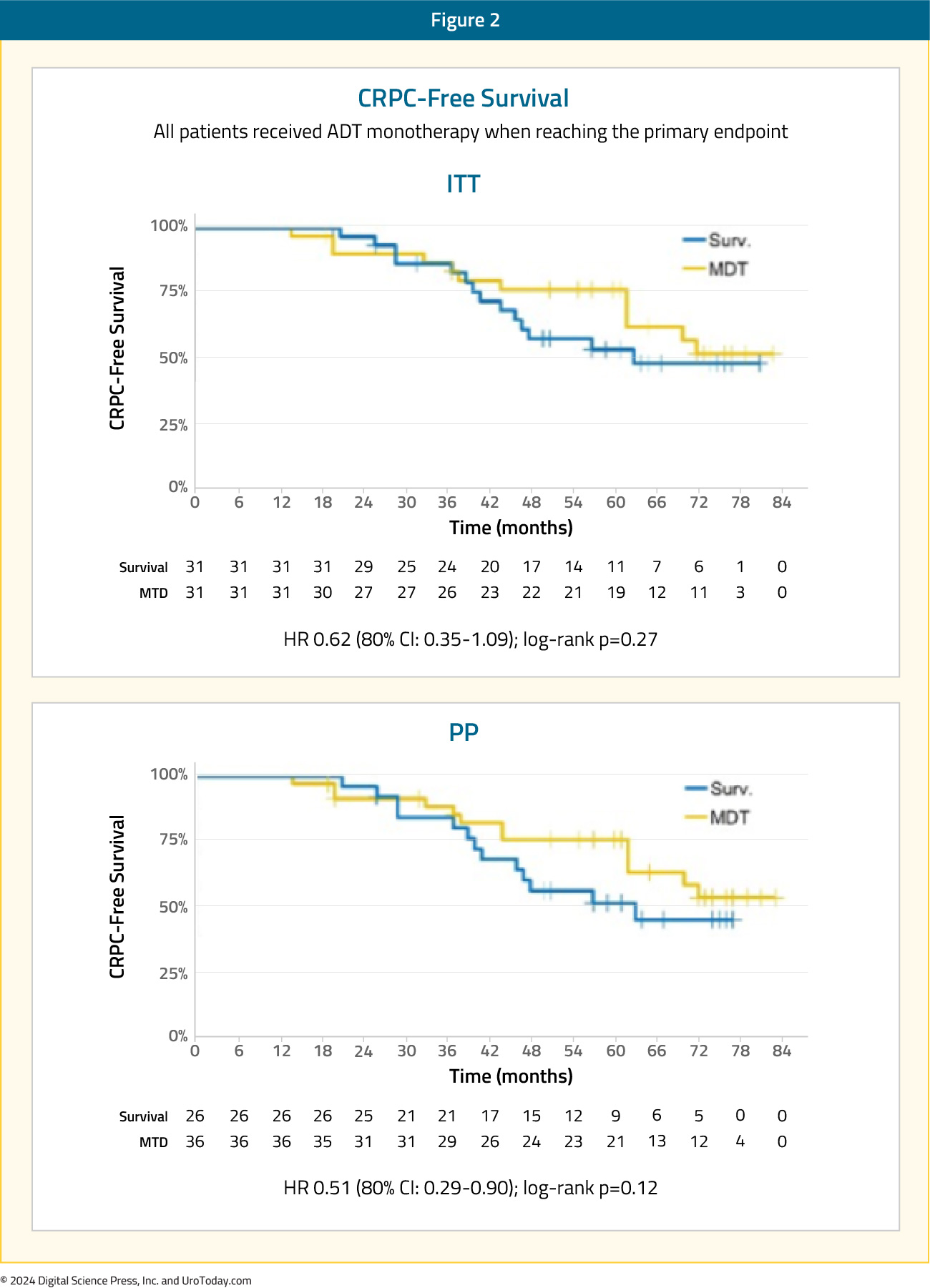
The secondary endpoint of 5-year CRPC-free survival was 53% in subjects under surveillance and 76% in those receiving MDT (HR 0.62, 80% CI: 0.35-1.09):2
The ORIOLE trial was a randomized phase II trial of men with recurrent oligometastatic hormone-sensitive prostate cancer (up to three sites). Between 2016 and 2018, 80 men were screened, of which 54 men had 1 to 3 metastases detectable by conventional imaging and had not received ADT within 6 months of enrollment or 3 or more years total. These 54 men were randomized in a 2:1 fashion to receive SBRT or observation. The primary outcome was disease progression at 6 months, defined by a serum PSA increase, radiographic progression on conventional imaging, symptomatic progression, ADT initiation for any reason, or death.
After a median follow-up of 19 months, disease progression at six months occurred in 19% of patients in the SBRT arm versus 61% of patients in the observation arm (p = 0.005). Patients in the SBRT treatment arm had superior median progression-free survival rates (median: not reached versus 5.8 months; HR: 0.30; 95% CI: 0.11–0.81; p = 0.002):
Secondary to the blinding of the investigative team to the PSMA-targeted PET data during treatment planning, 16 of 36 men treated with SBRT had baseline PET-avid lesions that were not included in the treatment fields. The proportion of men with no untreated lesions with progression at 6 months was 1 of 19 (5%) compared with 6 of 16 (38%) for those with any untreated lesions (p = 0.03). The median progression free survival was unreached among men with no untreated lesions vs 11.8 months among participants with any untreated lesions (HR, 0.26; 95% CI, 0.09-0.76; p = 0.006):3
As such, this was among the first data to suggest the importance of treating all PSMA PET-visible lesions for maximal oncologic benefit in the oligometastatic mHSPC space.
ORIOLE + STOMP: Pooled DataIn 2022, pooled data from the ORIOLE and STOMP trials demonstrated that MDT improves progression free survival from 5.9 months to 11.9 months (HR: 0.44, p<0.001), however without any significant improvements seen in radiographic progression-free survival, time to castration-resistant disease, or overall survival:4

SABR-COMET was a randomized, open-label phase II study of patients with oligometastatic disease (up to five sites) between February 2012 and August 2016. This trial was not restricted to patients with prostate cancer and also included lung, breast, and colorectal cancer patients. Of the 99 patients in this trial, 18 (18.2%) had prostate cancer. After stratifying by the number of metastases (1–3 versus 4–5), patients were randomized in a 1:2 fashion to receive either palliative standard of care alone or standard of care plus SBRT.
At a median follow-up of 5.7 years, the primary outcome of overall survival was superior for SBRT-treated patients. The 8-year overall survival rates were 27% and 14% in the intervention and control arms, respectively (HR: 0.50, 95% CI: 0.30–0.84, p = 0.008). The 8-year progression-free survival estimates were 21% and 0%, respectively (HR: 0.45, 95% CI: 0.28–0.72, p < 0.001):

The rates of grade ≥2 acute or late toxic effects were 30% versus 9% (p = 0.019), and the FACT-G quality of life scores declined over time in both arms, but with no differences in quality-of-life scores between the study arms.5
Combination of MDT + Systemic Therapy for Recurrent Oligometastatic Prostate Cancer
EXTENDThe EXTEND trial was a single center, phase II randomized controlled trial of 87 oligorecurrent men, mostly with mHSPC (>90%), who were randomized 1:1 to intermittent hormone therapy +/- MDT (definitive radiation therapy to all sites of disease). All patients had ≤5 metastases, as defined by conventional imaging (75%) or fluciclovine PET/CT (25%). A planned break in hormone therapy occurred 6 months after enrollment, after which hormone therapy was withheld until progression. At a median follow-up of 22 months, progression free survival was improved in the combined therapy arm (HR: 0.25, 95% CI: 0.12 – 0.55, p < 0.001). Significantly, ‘eugonadal’ progression free survival was also improved with this combination approach (HR: 0.32, p = 0.03):6
SATURN is a phase II trial of 28 men with oligorecurrent extra-pelvic metastases on PSMA-PET/CT following initial treatment with radical prostatectomy. Patients were treated with 6 months of ‘androgen annihilation therapy’, defined as leuprolide + abiraterone acetate/prednisone + apalutamide. After the 1st month of this systemic therapy, patients received SBRT to all metastases with or without radiotherapy directed to the prostate bed and pelvic lymph nodes. Results of the primary endpoint, the percentage of patients who maintained PSA <0.05 ng/mL six months after testosterone recovery to ≥150 ng/dL, were presented at ASCO GU 2024. Overall, 50% of patients maintained a PSA <0.05 ng/mL six months after testosterone recovery. After a median follow-up of 20 months, the median progression free survival was 19.3 months. Moreover, 81% of patients recovered eugonadal testosterone levels, at a median of 9.4 months from the start of systemic therapy, and the median eugonadal progression free survival was 11.4 months. Grade 3 adverse events related to androgen therapy were observed in 21% of patients, and SBRT had 7.7% grade 2 and no grade 3 toxicity.9
Key Ongoing Trials of MDT + Systemic TherapyThere are several important ongoing trials combining systemic therapy with the site specific control offered by MDT. The ADOPT trial is an ongoing phase III trial that is randomizing 280 patients with evidence of recurrent oligometastatic disease (≤4 lesions) on PSMA-PET/CT 1:1 to either MDT (radiotherapy) alone or MDT + 6 months of ADT. The primary endpoint of this trial is 30 month metastases progression free survival, with key secondary endpoints including overall survival, adverse events, and quality of life outcomes:7

NRG GU011 (PROMETHEAN) is a randomized phase II trial of SBRT with or without relugolix for early PET-detected recurrent oligometastatic prostate cancer. Eligible patients are those with biochemical recurrence following prior curative intent radiation or surgery for localized prostate cancer, PSA < 10 ng/mL, negative conventional imaging, and 1–5 PET-visible metastases (≥1 extra-pelvic). The primary endpoint is conventional imaging-based radiographic progression free survival. Key secondary endpoints include PET-based radiographic progression free survival, overall survival, and quality-of-life outcome measures:

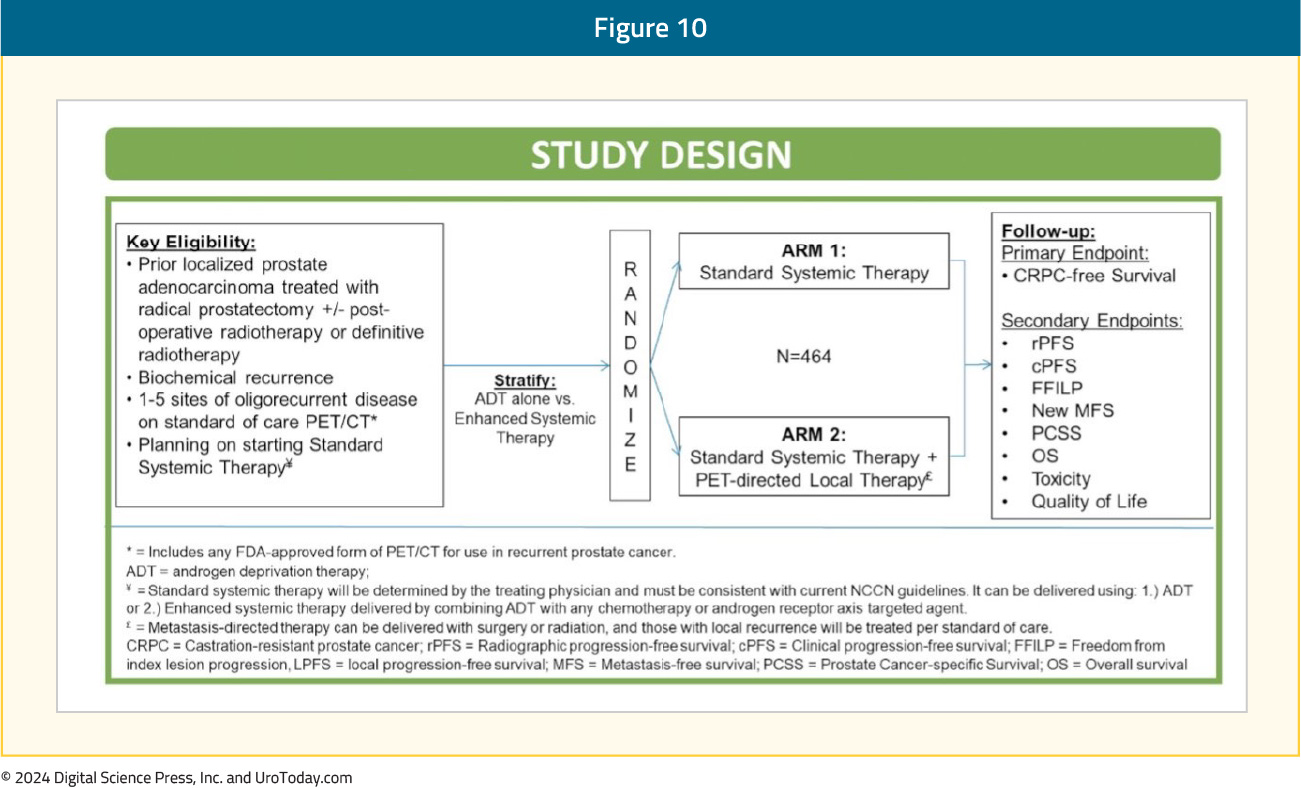
The VA STARPORT study is a phase II/III randomized trial that evaluates the value of adding PET-directed local therapy to standard systemic therapy for biochemically recurrent patients with evidence of ≤5 metastatic lesions on PSMA-PET/CT. PET-directed local therapy is defined as surgery or radiation to all visible metastases and any prostate/prostatectomy bed local recurrences. Systemic therapy will be administered indefinitely, consistent with current guidelines. The primary study endpoint is castrate-resistant prostate cancer-free survival. Key secondary endpoints include clinical and radiographic progression free survival, overall survival, toxicity, and quality of life outcomes:
PERSIAN is a randomized phase II trial of recurrent oligometastatic, hormone-sensitive patients (<5 non-visceral lesions). Eligible patients will undergo 1:1 randomization to apalutamide + ADT +/- SBRT to all visible lesions. The primary outcome is 6-month complete biochemical response. The trial design and key secondary endpoints are illustrated below:

The POSTCARD GETUG P13 is a French randomized phase II trial that is randomizing patients with recurrent oligometastatic disease following primary local therapy with curative intent to either SBRT alone (to all visible metastases) or SBRT + durvalumab. Oligometastasis is defined as ≤5 bone or lymph node metastases on 68Ga-PSMA PET/CT or ≤3 bone or lymph node metastases on 18F-choline PET/CT. The primary endpoint is two-year progression-free survival, with key secondary endpoints of androgen deprivation therapy free survival, quality of life, toxicity, prostate cancer specific survival, overall survival, and immune response.8
Incorporating PSMA-PET/CT into the MDT Paradigm
The next ‘frontier’ of MDT trials is incorporating PSMA-PET/CT for staging oligometastatic patients and determining eligibility for MDT. Given the increased sensitivity of PSMA-PET/CT compared to conventional imaging,10 it is likely that this may lead to a ‘stage migration’ phenomenon, where future cohorts of oligometastatic prostate cancer patients, matched for the same number of metastatic lesions, have more favorable prognoses. However, there has been a shift away from defining the ‘upper limit’ of oligometastatic disease by the number of metastatic lesions. A recent survey of participants from the ESTRO-ASTRO consensus conference demonstrated that the majority of respondents concur that the ‘upper limit’ of oligometastatic disease should be defined by the ability to safely deliver curative intent metastasis-directed radiotherapy and not by the number of metastatic lesions.
Existing evidence suggests that PSMA-PET/CT provides additional information in patients with evidence of recurrent oligometastatic disease on conventional imaging. In the ORIOLE trial, as highlighted above, pre-treatment 18F-DCFPyL-PET/CT was performed in all patients assigned to the MDT arm (n = 36). Of the 36 patients treated with SBRT, 16 (44.4%) had baseline PET-avid lesions that were not included in the treatment fields. These patients had significantly worse 6-month progression rates of 38% compared to those without untreated lesions (5%; p = 0.03). Furthermore, those with untreated sites of disease had higher rates of new metastases (per conventional imaging) at 6 months (62.5% versus 15.8%, p = 0.006), and worse median distant metastasis-free survival of 6 versus 29 months (HR: 0.19; 95% CI: 0.07–0.54, p < 0.001). These results highlight the importance of targeting all sites of disease, and the value of PSMA PET/CT for defining this with increased sensitivity compared to conventional imaging.3
A single arm phase II study evaluated the role of MDT (SBRT or surgery) in 37 patients with a rising PSA (0.4–3 ng/ml) following maximal local therapy (radical prostatectomy + adjuvant/salvage radiotherapy) who had not received prior salvage hormonal therapy and had negative conventional imaging, but evidence of oligometastasis on 18F-DCFPyL PET-MR/CT. Ten and 27 patients underwent surgery and SBRT, respectively. At a median follow-up of 16 months, the overall response rate was 60%, including 22% who had biochemical ‘no evidence of disease. One (2.7%) grade 3 toxicity (intra-operative ureteric injury) was observed.11
MDT for De Novo Oligometastatic Hormone-sensitive Prostate Cancer
There are numerous ongoing trials evaluating the role of MDT in combination with systemic therapy in the de novo oligometastatic hormone-sensitive setting. In this section, we will highlight key trials in this space.
SOLAR (NCT03298087) is a single arm phase II trial of 28 patients with de novo M1a/b disease and 1–5 radiographically visible M1 lesions (majority detected via PSMA PET-CT) who all underwent radical local treatment, intensified systemic therapy for six months (leuprolide, abiraterone acetate with prednisone, apalutamide), and metastasis-directed SBRT. Radical local therapy was either radical prostatectomy (n = 12) with lymph node dissection and postoperative radiotherapy (for ≥pT3a, N1, or positive margins) or radical radiotherapy (n=12) directed to the prostate, seminal vesicles, and pelvic lymph nodes. The initial results of this trial were presented at GU ASCO 2024. At a median follow-up of 30 months, 62% of patients completed all planned systemic therapy without dose modification. Of the 22 patients with >6 months follow-up after testosterone recovery, 86% remained free of any progression, defined as an undetectable serum PSA after radical prostatectomy or <2 ng/ml after radical radiotherapy. Grade 2 and 3 toxicities for primary tumor therapy were 46% and 4%, respectively. There were no grade 2–3 toxicities relating to SBRT.12
PLATON (NCT03784755) is a two-arm phase II RCT exploring sequential treatment with ADT +/- chemotherapy followed by cytoreductive prostatectomy or external beam radiotherapy and then SBRT for oligometastases in 410 men. The comparator arm consists of systemic therapy alone, although low-volume men will be allowed to receive conventional local prostate radiotherapy. The primary study outcome is failure-free survival:

IP2-ATLANTA (NCT03763253) is a three-arm phase II randomized trial exploring sequential systemic, local, physical cytoreductive therapy and finally SBRT versus standard of care in 918 men with any-volume metastatic disease. All patients will receive doublet systemic therapy (ADT + docetaxel or enzalutamide or abiraterone). Patients in experimental arm 1 will receive minimally invasive ablative therapy +/- lymph node dissection, whereas those randomized to experimental arm 2 will receive local external beam radiotherapy +/- lymph nodes or radical prostatectomy +/- pelvic lymph node dissection. Patients in both experimental arms will further receive SBRT to oligometastases following receipt of of systemic and local treatment. Patients in the control arm will receive planned systemic therapy only, although patients with low-volume metastases will be eligible for prostate external beam radiotherapy:13

Finally, TERPs (NCT05223803) is a phase II trial of patients with de novo oligometastatic disease (<3 on conventional imaging or <5 on PET/CT) who will be randomized 1:1 to best systemic therapy + primary prostate radiotherapy +/- MDT (SBRT). The primary study endpoint is two-year failure-free survival.14
Conclusions and Future Directions
MDT has emerged as a guideline-recommended treatment option for prostate cancer patients with oligometastatic disease. The evidence to date suggests that MDT can be used in lieu of systemic therapy to delay time-to-hormone therapy or in combination with systemic therapy as a consolidative measure. The latest NCCN guidelines recommend considering SBRT for MDT in oligometastatic patients when ablation is the goal or in oligometastatic patients with limited progression or limited residual disease on otherwise effective systemic therapy (i.e., consolidation), where progression free survival is the goal.
The next ‘frontier’ of clinical trials for MDT will be evaluating its efficacy and safety in patients with PSMA-PET/CT-defined oligometastases and defining its role in the de novo metastatic hormone-sensitive setting. The results of these exciting trials will become available over the next few years.
Published August 2024
- Written by: Rashid K. Sayyid, MD MSc, University of Southern California, Los Angeles, CA & Zachary Klaassen, MD MSc, Wellstar MCG Health, Augusta, GA
- References:
- Foster CC, Pitroda SP, Weichselbaum RR. Definition, Biology, and History of Oligometastatic and Oligoprogressive Disease. Cancer J. 2020;26(2):96-9.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. J Clin Oncol. 2020;38:6_suppl.
- Philips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-9.
- Deek MP, van der Eecken K, Sutera P, et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022;40(29):3377-82.
- Harrow S, Palma DA, Olson R, et al. Stereotactic Radiation for the Comprehensive Treatment of Oligometastases (SABR-COMET): Extended Long-Term Outcomes. Int J Radiat Oncol Bio Phys. 2022;114(4):611-6.
- Tang C, Sherry AD, Haymaker C, et al. Addition of Metastasis-Directed Therapy to Intermittent Hormone Therapy for Oligometastatic Prostate Cancer: The EXTEND Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023;9(6): 825-34.
- Janssen J, Staal FHE, Brouwer CL, et al. Androgen Deprivation therapy for Oligo-recurrent Prostate cancer in addition to radioTherapy (ADOPT): study protocol for a randomised phase III trial. BMC Cancer. 2022;22(1):482.
- Roge M, Pointreau Y, Sargos P, et al. Randomized phase II trial in prostate cancer with hormone-sensitive OligometaSTatic relapse: Combining stereotactic ablative radiotherapy and durvalumab (POSTCARD GETUG P13): Study protocol. Clin Transl Radiat Oncol. 2023;40:100613.
- Nikitas J, Rettig M, Shen J, et al. Systemic and tumor-directed therapy for oligorecurrent metastatic prostate cancer (SATURN): Primary endpoint results of a phase II clinical trial. J Clin Oncol. 2024;42:Number 4_suppl.
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208-16.
- Glicksman RM, Metser U, Vines D, et al. Curative-intent Metastasis-directed Therapies for Molecularly-defined Oligorecurrent Prostate Cancer: A Prospective Phase II Trial Testing the Oligometastasis Hypothesis. Eur Urol. 2021;80(3):374–82.
- Nickols NG, Tsai S, Kane N, et al. Systemic and tumor-directed therapy for oligometastatic prostate cancer (SOLAR): A phase II trial for veterans with de novo oligometastatic prostate cancer. J Clin Oncol. 2024;42:Number 4_suppl.
- Connor MJ, Shah TT, Smigielska K, et al. Additional Treatments to the Local tumour for metastatic prostate cancer-Assessment of Novel Treatment Algorithms (IP2-ATLANTA): protocol for a multicentre, phase II randomised controlled trial. BMJ Open. 2021;11(2):e042953.
- Rana ZH, Helie N, Eggleston C, et al. Phase 2 randomized total eradication of metastatic lesions following definitive radiation to the prostate in de novo oligometastatic prostate cancer (TERPs) trial. J Clin Oncol. 2023;41:6_suppl.
PARP Inhibitor Therapy for Prostate Cancer Patients: Emerging Combinations
Introduction
Poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors are drugs that prevent the repair of DNA single-stranded breaks and promote their conversion to double-stranded breaks resulting in a synthetic lethality.1 These drugs have demonstrated promising results for the treatment of metastatic castrate-resistant prostate cancer (mCRPC) patients who experience disease progression following prior androgen receptor pathway inhibitor (ARPI) and/or taxane-based chemotherapy.There is growing interest in combining these agents with other classes of drugs that may have synergistic mechanisms of action. A prime example of this is the use of combination PARP inhibitors and APRIs, with ARPIs inhibiting the transcription of specific homologous recombination repair (HRR) genes, inducing an HRR deficiency-like state, which potentiates PARP inhibitor activity, and, conversely, PARP inhibitors upregulating androgen receptor signaling, enhancing ARPI activity.2-4 This has culminated in the approval of three PARP inhibitor/ARPI combinations by the US Food and Drug Administration (FDA) for the treatment of mCRPC patients in the first line setting:
- Olaparib plus abiraterone for BRCA1/2-mutated patients5
- Niraparib plus abiraterone for BRCA1/2-mutated patients6
- Talazoparib plus enzalutamide for HRR-mutated patients7
PARP Inhibitors + Radium-223
Olaparib + Radium-223For patients with bone metastases, it has been theorized that the combination of a PARP inhibitor and radium-223 may have synergistic mechanisms of action. PARP inhibitors have shown efficacy as radiosensitizing agents which may promote the efficacy of radium-223, an α-emitting radioisotope that induces DNA double-strand breaks leading to cell death. This formed the foundation for the COMRADE trial, an open-label, multi-center, phase 1/2 study trial to test the safety and efficacy of radium-223 and olaparib. This trial included men with mCRPC who had ≥2 bone metastases without evidence of concurrent visceral metastases or lymphadenopathy > 4 cm.
The phase 1 portion of the study employed a 3+3 dose escalation design with fixed-dose radium-223 (55 kBq/kg IV every 4 weeks x 6) and escalating doses of olaparib. The dose level 1 (DL1) for was olaparib 200 mg PO BID while DL2 was 300 mg PO BID. In phase 1, the primary objective was to determine the recommended phase 2 dose (RP2D) for the randomized portion of the study, which was found to be 200 mg BID for olaparib. No dose limiting toxicities were observed at either DL1 or DL2. However, 5 of 6 patients enrolled at DL2 required dose reduction. Assessing secondary objectives, the authors found that the PSA response and alkaline phosphatase response rates were 16.7% (n=2) and 67% (n=8), respectively. At a median follow-up of 6.5 months, the 6 months rPFS was 58%, and the 12 months OS was 56%. Based on these results, the investigators concluded that olaparib can be safely combined with radium-223 at the RP2D of 200 mg orally twice daily with fixed dose radium-223.8
Niraparib + Radium-223
Utilizing a similar treatment strategy to that seen in the COMRADE trial, the combination of niraparib and radium-223 was evaluated in the phase 1b trial, NiraRad. This trial included 30 men with progressive mCRPC following ≥1 line of an ARPI and had evidence of bone metastases without bulky visceral disease and no documented BRCA1/2 alterations. The niraparib dose was escalated in combination with standard dosing of Radium-223 using a time-to-event continual reassessment method. The investigators determined that for patients with prior chemotherapy exposure, the maximum tolerated dose (MTD) for niraparib was 100 mg, whereas the MTD for chemotherapy-naïve patients was 200 mg. The median rPFS for all patients included in analysis was 7.1 months with an estimated 6-month rPFS of 51%.9
PARP Inhibitors + 177Lu-PSMA-617
177Lu-PSMA-617 delivers significant beta radiation to PSMA-expressing tumors causing single strand DNA breaks, which are typically repaired by PARP-dependent pathways. Blocking the PARP enzyme could have a synergistic mechanism of action by converting DNA single strand breaks to lethal double strand breaks via replication fork collapse. In the LuPARP trial presented at ASCO 2023, the investigators hypothesized that olaparib would promote the radiosensitization of 177Lu-PSMA-617, resulting in intensification of DNA damage and, thus, improved efficacy.
The LuPARP phase 1 trial schema was as follows:
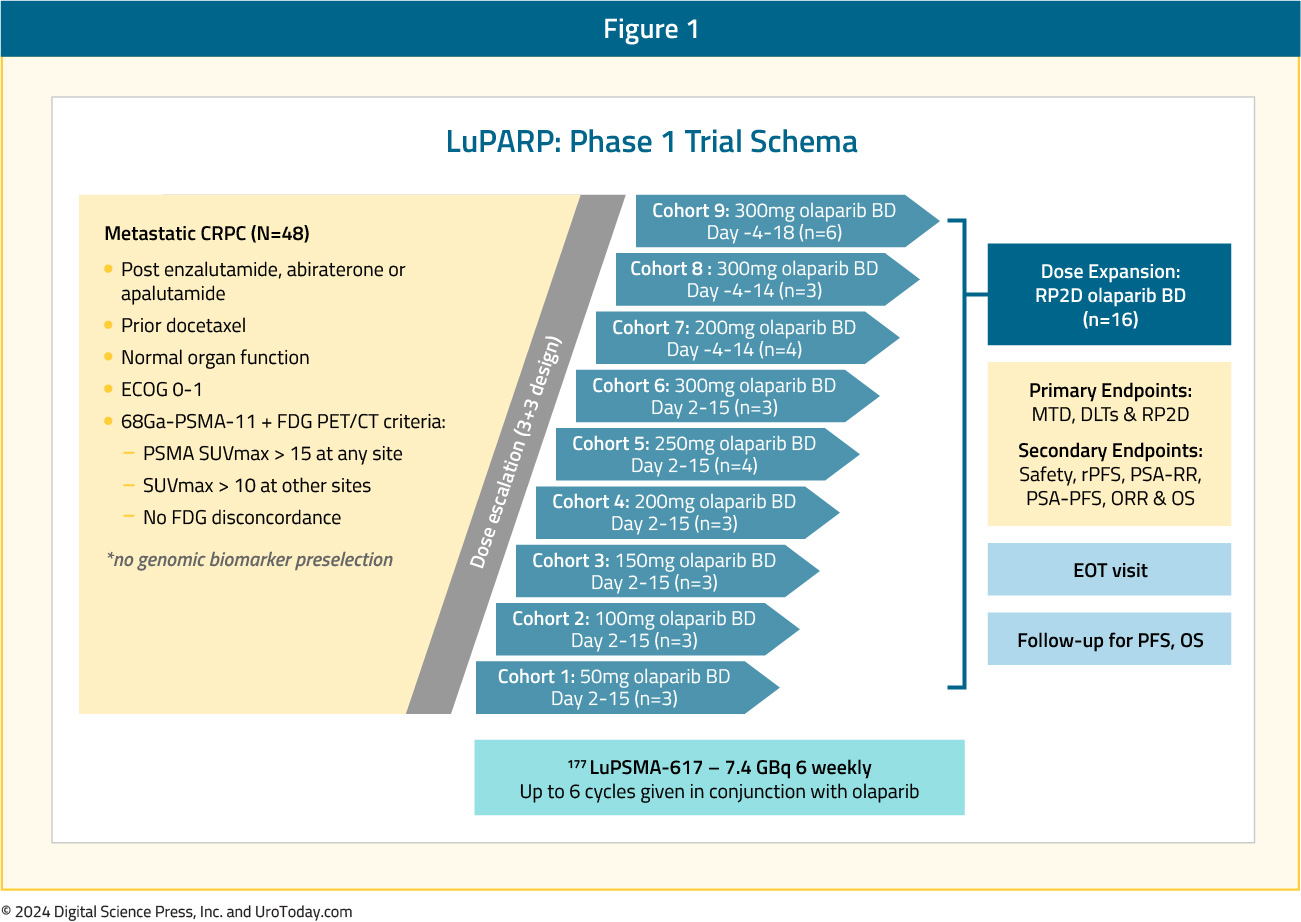
This trial included 48 patients with mCRPC, and all eligible patients had received a prior ARPI and docetaxel. All patients underwent a 68Ga-PSMA-11 plus an FDG-PET/CT with the following inclusion criteria:
- PSMA SUVmax >15 at any site
- SUVmax >10 at other sites
- No FDG discordance
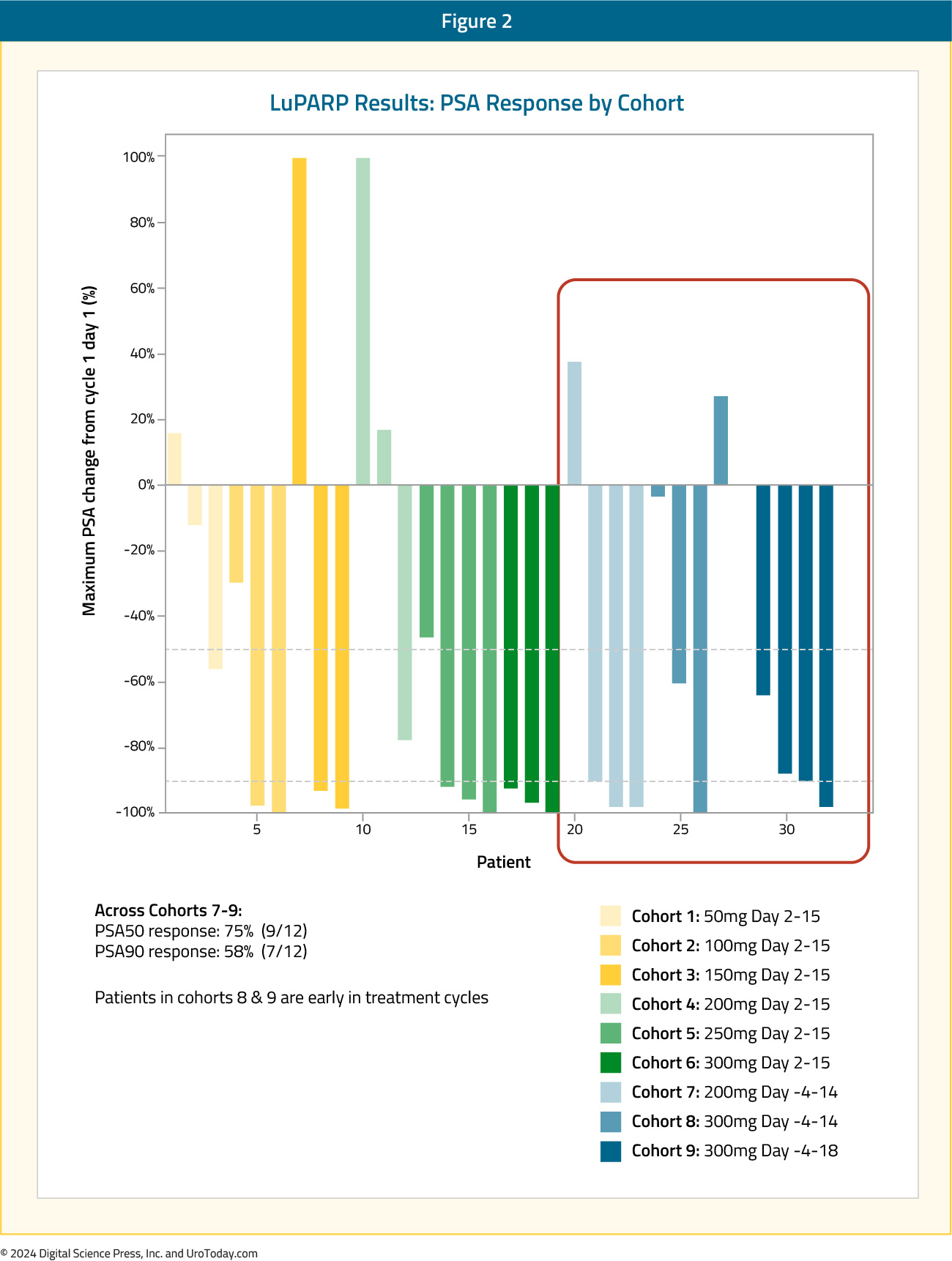
From an efficacy standpoint, 177Lu-PSMA-617 in combination with olaparib demonstrated promising activity: in the overall cohort (i.e., Cohorts 1 to 9), the PSA50 and PSA90 response rates were 66% and 44%, respectively. The objective response rate (ORR) by RECIST v1.1 criteria was 78%.10 Compared to the results of the TheraP and VISION trials, the PSA50 responses were identical to those from TheraP (66%) and higher than those in VISION (46%).11,12 The PSA90 response of 44% in LuPARP was slightly higher than that in TheraP (38%).
Moreover, early results from Cohorts 7-9 were promising with PSA50 and PSA90 responses of 75% and 58%, respectively. However, results from this Phase 1 trial are not designed, nor powered, to assess efficacy outcomes.
PARP Inhibitors + Immune Checkpoint Inhibitors
While immunotherapy has shown limited success in the mCRPC disease space, it is hypothesized that the increased cellular DNA damage induced by PARP inhibitors may lead to increased immune priming and subsequently promote immune cell infiltration. This has served as the rationale for emerging trials of combination PARP inhibitors and immune checkpoint inhibitors.Rucaparib + Nivolumab
The CheckMate 9KD trial has evaluated the combination of rucaparib and nivolumab in two cohorts:
- Cohort A1: Post-chemotherapy mCRPC (1–2 taxanes and ≤2 ARPIs)
- Cohort A2: Chemotherapy-naïve mCRPC (Received prior ARPI)
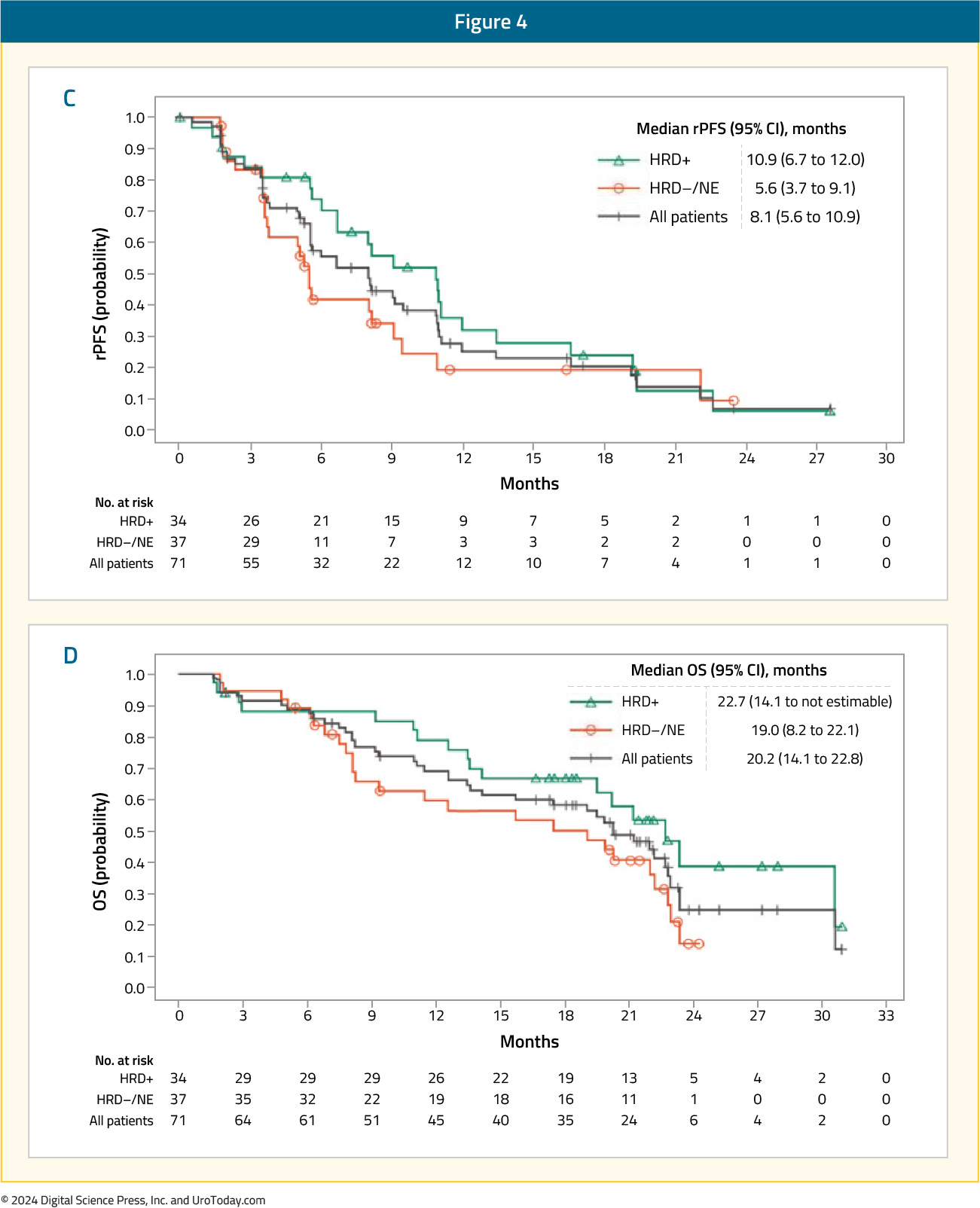
Among patients in Cohort A1 (n=58), the ORR was 10.3% in the overall cohort. Superior ORRs were observed in the HRD-positive (17.2%) and BRCA1/2-positive tumors (33.3%). PSA50 responses were observed in 12% of patients in the overall cohort, compared to 18% and 42% of HRD-positive and BRCA1/2-positive tumors, respectively. Median rPFS ranged between 4.9 and 5.8 months, whereas OS ranged between 13.9 and 15.4 months.

As expected, response rates and survival outcomes were superior in the less heavily pre-treated Cohort A2 (n=39). The ORR was 15.4% in the overall cohort, with ORRs of 25% and 33.3% in the HRD-positive and BRCA1/2-positive tumors, respectively. PSA50 responses were observed in 27.3% of patients in the overall cohort, compared to 42% and 85% of HRD-positive and BRCA1/2-positive tumors, respectively. Median rPFS ranged between 8.1 and 10.9 months, whereas OS ranged between 20.2 and 22.7 months.
In cohorts A1 and A2, respectively, the most common any-grade and grade 3–4 treatment-related adverse events were nausea (41%) and anemia (14–21%). Approximately 25% of patients discontinued treatment secondary to adverse events.13
Olaparib + Pembrolizumab
Cohort A of the phase 1b/2 KEYNOTE-365 study enrolled patients with molecularly unselected, docetaxel-pretreated mCRPC whose disease progressed within 6 months of screening. In this trial, 102 patients received pembrolizumab 200 mg IV every 3 weeks + olaparib 400 mg capsule or 300 mg tablet orally twice daily. Patients could have received one chemotherapy agent other than docetaxel for mCRPC and ≤2 ARPIs. The primary endpoints were PSA50 response rates, ORR, and safety.
A PSA50 response was observed in 15% of patients. The confirmed ORR was 8.5% (5 partial responses) among patients with measurable disease.

The median rPFS was 4.5 months, and the median OS was 14 months. Treatment-related adverse events were observed in 91% of patients. Grade 3–5 events occurred in 48% of patients (6% deaths), most commonly anemia (27%), fatigue (6%), and neutropenia (5%).14
This combination of olaparib + pembrolizumab was next assessed in the open-label, phase III KEYLYNK-010 trial that randomized mCRPC patients that had progressed on one prior ARPI and docetaxel in a 2:1 fashion to olaparib + pembrolizumab versus the alternate ARPI (i.e., if had received abiraterone, given enzalutamide and vice versa). The dual primary endpoints were rPFS and OS. This trial included 793 patients of whom 529 and 264 were randomized to olaparib + pembrolizumab and an alternate ARPI, respectively. There was no significant difference in rPFS (median: 4.4 versus 4.2 months; HR: 1.02, 95% CI: 0.82 – 1.25, p=0.55) or OS between the two treatment arms (median 15.8 versus 14.6 months; HR: 0.94, 95% CI: 0.77 – 1.14, p=0.26).
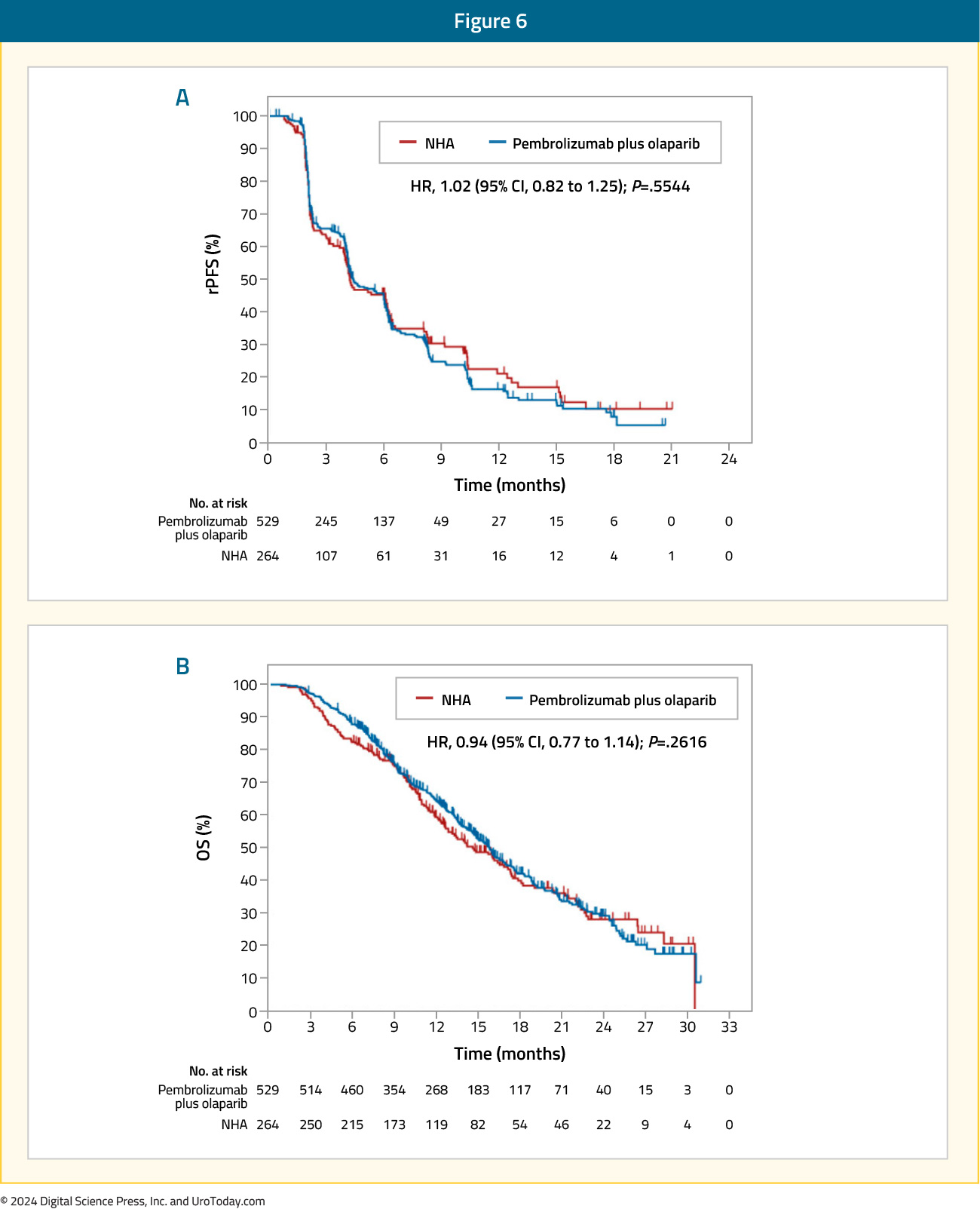
Grade 3 treatment-related adverse events were more common with olaparib + pembrolizumab (35% versus 9%), with events leading to treatment discontinuation occurring in 11% and 1.6% of patients in the intervention and control arms, respectively. The most common grade ≥3 adverse events with olaparib + pembrolizumab were anemia (20%), fatigue (3%), and asthenia (2.3%).15
Olaparib + Durvalumab
In a single arm phase II trial, the combination of durvalumab 1,500 mg IV every 4 weeks and olaparib 300 mg twice daily was evaluated in 17 mCRPC patients with disease progression following prior ARPI. Overall, 9/17 (53%) patients had a PSA50 response, with 4 of these 9 patients having a radiographic response. The median rPFS of patients with DDR gene alteration was 16.1 months, with a 12-months PFS probability of 83.3%, compared to 36.4% in those without mutations (p=0.031). The most common treatment-related grade 3 or 4 adverse events were anemia (24%), lymphopenia (12%), infection (12%), and nausea (12%).16
Talazoparib + Avelumab
The JAVELIN PARP Medley trial is a phase 1b/2 basket trial evaluating the combination of talazoparib and avelumab in patients with advanced solid tumors, including mCRPC patients with and without HHR alterations (n=21). Patients received avelumab 800 mg every 2 weeks plus talazoparib 1mg once daily. In the overall cohort, PSA responses were observed in 2/21 patients, and in the HRR positive mCRPC cohort, the ORR was 11.1%.17
PARP Inhibitors + Bipolar Androgen Therapy
Prostate cancer cells can develop resistance to androgen ablation through an adaptive marked upregulation of androgen receptors over time in response to a low-androgen milieu. This upregulation can make these cells vulnerable to supraphysiologic testosterone exposure. Bipolar Androgen Therapy (BAT) has been proposed as a technique to overcome AR therapeutic resistance. Rapid cycling between polar extremes of supraphysiologic and near-castrate serum testosterone in asymptomatic men with mCRPC has proven to be safe and effective.18Supraphysiologic androgen levels have been shown to induce double-strand DNA breaks and suppress the expression of genes involved in the DNA repair process.19,20 This has served as the rationale for evaluating the combination of olaparib and BAT in a single arm phase II trial. Thirty-six patients with mCRPC and disease progression following abiraterone and/or enzalutamide received olaparib 300 mg twice daily plus BAT (testosterone cypionate/enanthate 400 mg every 28 days with ongoing androgen deprivation). A PSA50 response was observed in 11/36 patients (31%) at 12 weeks, and the median rPFS in the intent-to-treat cohort was 13 months. The most frequently observed treatment-related adverse events were gastrointestinal related and fatigue. Five patients had grade ≥3 treatment-related adverse events, including one stroke (Grade 4) and one myocardial infarction (Grade 5).21
PARP Inhibitors + Chemotherapy
The combination of the low dose oral PARP inhibitor veliparib (ABT-888) and temozolomide for docetaxel pre-treated mCRPC patients was evaluated in a single-arm, open-label, pilot study published by Hussain et al. in 2014. This trial included 26 patients with a median baseline PSA of 170 ng/ml. A PSA response was observed in 2 patients (8%), with a further 13 having stable PSA levels. The median PFS was 9 weeks, and the median OS was 40 weeks. Grade 3/4 adverse events occurred in >10 % of patients include thrombocytopenia (23 %) and anemia (15 %).22PARP Inhibitors + Targeted Therapies
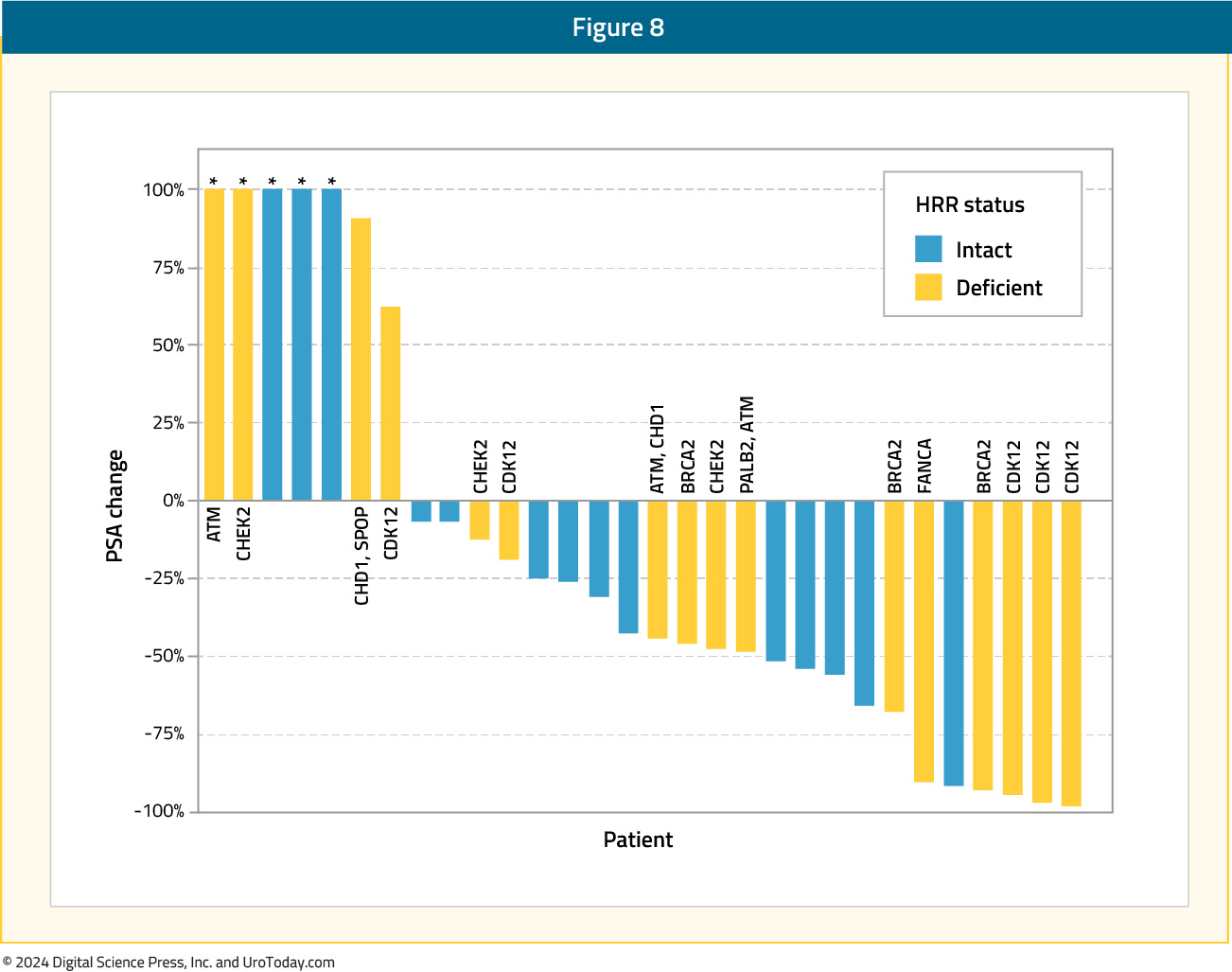
Olaparib + CediranibCediranib is a pan-vascular endothelial growth factor receptor inhibitor that suppresses the expression of HRR genes and increases sensitivity to PARP inhibition in preclinical models.23 In an open-label phase II trial, patients with progressive mCRPC were randomly assigned to receive either cediranib 30 mg once daily plus olaparib 200 mg twice daily versus olaparib 300 mg twice daily alone. In the intention-to-treat cohort of 90 patients, the median rPFS was 8.5 months in the combination arm versus 4 months in the PARP inhibitor monotherapy arm (HR 0.62; 95% CI: 0.39–0.97, p=0.036). Among patients with HRR-deficient mCRPC, the median rPFS was 10.6 months with combination treatment versus 3.8 months with olaparib monotherapy. In the subset of patients with BRCA2-mutated mCRPC, median rPFS was 13.8 months in the combination arm versus 11.3 months in the olaparib only arm. Grade 3–4 adverse events occurred in 61% of patients in the combination arm, compared to 18% of patients in the monotherapy arm.24
Olaparib + Ceralasterib
In an in vitro study, the combination of olaparib and the ataxia telangiectasia and Rad3-related protein (ATR) inhibitor, ceralasterib, was shown to selectively cause cell death in ATM-deficient cells.25 This served as the basis for the TRAP trial, a two-cohort study of mCRPC patients with HRR mutations (BRCA1/2 or ATM; n=35) and another without HRR mutations (n=12). All patients had progressed on ≥1 prior mCRPC therapy with no prior PARP inhibitors or platinum chemotherapy. In this study, olaparib was administered twice daily at a standard dose, and ceralasterib was administered daily on days 1¬–7 of a 28-day cycle. The primary endpoint was disease response (confirmed PSA50 or RECIST response). The response rate in the HRR cohort was 33%, compared to 11% in the HRR negative cohort, including 21% of patients experiencing a grade 3 treatment-related adverse event (no grade 4–5 events).26

Conclusions and Future Trials
PARP inhibitors are an exciting class of drugs with a unique mechanism of action that lends itself to potential synergistic combinations with other classes of drugs. To date, the only combination to receive regulatory approval is that of PARP inhibitors + ARPIs; however, numerous exciting combinations continue to emerge. Additionally, given their success in the mCRPC disease space, there is increased interest in evaluating such combinations in earlier disease stages, including the high-risk localized and the metastatic hormone-sensitive settings. Summarized in the table below are select trials of PARP inhibitor combination therapy across the prostate cancer spectrum.

Published March 2024
- Written by: Zachary Klaassen, MD, MSc Associate Professor of Urology Urologic Oncologist Medical College of Georgia, Georgia Cancer Center Augusta, GA and Rashid Sayyid, MD, MSc Urologic Oncology Fellow University of Toronto Toronto, Ontario, Canada
- References:
- Xie T, Dickson K, Yee C, et al. Targeting Homologous Recombination Deficiency in Ovarian Cancer with PARP Inhibitors: Synthetic Lethal Strategies That Impact Overall Survival. Cancers (Basel). 2022;14(19):4621.
- Asim M, Tarish F, Zecchini HI, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun. 2017; 8:374.
- Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012; 2:1134-49.
- Li L, Karanika S, Yang G, et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal. 2017;10: eaam7479.
- FDA approves olaparib with abiraterone and prednisone (or prednisolone) for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-abiraterone-and-prednisone-or-prednisolone-brca-mutated-metastatic-castration. Accessed on March 10, 2024.
- FDA approves niraparib and abiraterone acetate plus prednisone for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-and-abiraterone-acetate-plus-prednisone-brca-mutated-metastatic-castration. Accessed on March 10, 2024.FDA approves niraparib and abiraterone acetate plus prednisone for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-and-abiraterone-acetate-plus-prednisone-brca-mutated-metastatic-castration. Accessed on March 10, 2024.FDA approves niraparib and abiraterone acetate plus prednisone for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-and-abiraterone-acetate-plus-prednisone-brca-mutated-metastatic-castration. Accessed on March 10, 2024.
- FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate. Accessed on March 10, 2024.FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate. Accessed on March 10, 2024.FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate. Accessed on March 10, 2024.
- Pan E, Xie W, Ajmera A, et al. A Phase I Study of Combination Olaparib and Radium-223 in Men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) with Bone Metastases (COMRADE). Mol Cancer Ther. 2023;22(4):511-518.
- Quinn Z, Leiby B, Sopavde G, et al. Phase I Study of Niraparib in Combination with Radium-223 for the Treatment of Metastatic Castrate-Resistant Prostate Cancer. Clin Cancer Res. 2023;29(1):50-59.
- Sandhu S, Joshua AM, Emmett L, et al . LuPARP: Phase 1 trial of 177Lu-PSMA-617 and olaparib in patients with metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(16):Suppl 5005.
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1901-1103
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- Fizazi K, Retz M, Petrylak DP, et al. Nivolumab plus rucaparib for metastatic castration-resistant prostate cancer: results from the phase 2 CheckMate 9KD trial. J Immunother Cancer. 2022;10(8):e004761.
- Yu EY, Piulats JM, Gravis G, et al. Pembrolizumab plus Olaparib in Patients with Metastatic Castration-resistant Prostate Cancer: Long-term Results from the Phase 1b/2 KEYNOTE-365 Cohort A Study. Eur Urol. 2023;83(1):15-26.
- Antonarakis ES, Park SH, Goh JC, et al. Pembrolizumab Plus Olaparib for Patients With Previously Treated and Biomarker-Unselected Metastatic Castration-Resistant Prostate Cancer: The Randomized, Open-Label, Phase III KEYLYNK-010 Trial. J Clin Oncol. 2023;41(22):3839-3850.
- Karzai F, VanderWeele D, Madan RA, et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J Immunother Cancer. 2018;5(1):141.
- Yap TA, Bardia A, Dvorkin M, et al. Avelumab Plus Talazoparib in Patients With Advanced Solid Tumors: The JAVELIN PARP Medley Nonrandomized Controlled Trial. JAMA Oncol. 2023;9(1):40-50.
- Denmeade SR, Wang H, Agarwal N, et al. TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men With Castration-Resistant Metastatic Prostate Cancer. J Clin Oncol. 2021;39(12):1371-1382.
- Haffner MC, Aryee MJ, Toubaji A, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010;42(8):668-675.
- Chatterjee P, Schweizer MT, Lucas JM, et al. Supraphysiological androgens suppress prostate cancer growth through androgen receptor-mediated DNA damage. J Clin Invest. 2019;129(1):4245-4260.
- Schweizer MT, Gulati R, Yezefski T, et al. Bipolar androgen therapy plus olaparib in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2023;26(1):194-200.
- Hussain M, Carducci MA, Slovin S, et al. Targeting DNA repair with combination veliparib (ABT-888) and temozolomide in patients with metastatic castration-resistant prostate cancer. Invest New Drugs. 2014;32(5):904-912.
- Kaplan AR, Gueble SE, Liu Y, et al. Cediranib suppresses homology-directed DNA repair through down-regulation of BRCA1/2 and RAD51. Sci Transl Med. 2019;11:eaav4508.
- Kim JW, McKay RR, Radke MR, et al. Randomized Trial of Olaparib With or Without Cediranib for Metastatic Castration-Resistant Prostate Cancer: The Results From National Cancer Institute 9984. J Clin Oncol. 2023;41(4):871-880.
- Lloyd RL, Wijnhoven PWG, Ramos-Montoya A, et al. Combined PARP and ATR inhibition potentiates genome instability and cell death in ATM-deficient cancer cells. Oncogene. 2020;39(25):4869-4883.
- Reichert ZR, Devitt ME, Alumkal JJ, et al. Targeting resistant prostate cancer, with or without DNA repair defects, using the combination of ceralasertib (ATR inhibitor) and olaparib (the TRAP trial). J Clin Oncol. 2022;40(6):Supplement.
American Society of Clinical Oncology (ASCO) 2024 Bladder Cancer Updates
EV+P nearly doubled median PFS and OS versus platinum-based chemotherapy in patients with previously untreated locally advanced or metastatic urothelial cancer in the phase 3 EV-302 trial and is NCCN category 1 and ESMO guidelines preferred treatment option. PRO assessments included the
PARP Inhibitor Monotherapy for Prostate Cancer Patients
Introduction
Over the past decade, there have been significant advances in defining the genomic landscape of prostate cancer. The landmark study by Pritchard et al. published in The New England Journal of Medicine in 2016 demonstrated that germline DNA-repair gene mutations were present in approximately 12% of metastatic prostate cancer patients, most commonly BRCA2 (5.3%), CHEK2 (1.9%), and ATM (1.6%). Significantly, the frequency of such mutations increases across the prostate cancer spectrum – 2% in patients with NCCN localized low-to-intermediate risk tumors, 6% in those with localized high-risk tumors, and as high as 24% in patients with metastatic castrate-resistant prostate cancer (mCRPC).1 This is of utmost clinical importance as such mutations, both inherited and acquired (i.e., somatic), represent actionable clinical targets for drug therapy.
Poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors are drugs that prevent the repair of DNA single-stranded breaks and promote their conversion to double-stranded breaks leading to a synthetic lethality. These agents are most effective in homologous recombination repair (HRR)-deficient tumors (e.g., BRCA1/2), due to their compromised ability to repair DNA double strand breaks.2 In addition to breast and ovarian malignancies, PARP inhibitors have gained regulatory approval for the treatment of mCRPC patients:
- Rucaparib for BRCA1/2-mutated patients (FDA approved in 2020)3
- Olaparib for HRR-mutated patients (FDA approved in 2020)4
- Olaparib plus abiraterone for BRCA1/2-mutated patients (FDA approved in 2023)5
- Niraparib plus abiraterone for BRCA1/2-mutated patients (FDA approved in 2023)6
- Talazoparib plus enzalutamide for HRR-mutated patients (FDA approved in 2023)7
In this Center of Excellence article, we will provide an in-depth overview of the current evidence for PARP inhibitor monotherapy in prostate cancer, summarizing efficacy results from major trials and discussing the adverse event profile of these agents.
Current Evidence for PARP Inhibitor Monotherapy
Olaparib
TOPARP-A was a pivotal phase II trial of olaparib in mCRPC in which 50 patients were treated with olaparib 400 mg twice daily until disease progression.8 The primary endpoint was the composite response rate defined either as an objective response according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria, or a ≥ 50% reduction in prostate-specific antigen (PSA50), or a reduction in the circulating tumor-cell count from ≥ 5 per 7.5 ml of blood to < 5 per 7.5 ml. All patients had prior treatment with docetaxel and 49 (98%) with abiraterone or enzalutamide. Sixteen of 49 (33%) evaluable patients had a response. Overall, 14 of the 16 responders had homozygous deletions, deleterious mutations, or both in DNA-repair genes — including BRCA1/2, ATM, Fanconi’s anemia genes, and CHEK2.
This was followed by TOPARP-B, an open-label, phase II trial in which men with HRR-mutated mCRPC that had progressed on ≥1 taxane therapy were treated with olaparib 400 mg or 300 mg twice daily in a randomized fashion.9 The primary endpoint was identical to the TOPARP-A trial. A targetable HRR gene aberration was found in 161 of 592 (27.2%) patients who underwent a targeted next-generation tumor sequencing. However, sequencing could not be performed on 119 (17%) of consented patients because of insufficient or poor-quality tissue. The confirmed composite response rate was 54.3% in the 400 mg cohort and 39.1% in the 300 mg cohort (p=0.14). Median radiographic progression-free survival (rPFS) was 5.5 months (95% CI: 4.4 – 8.3) in the 400 mg cohort and 5.6 months (3.7 – 7.7) in the 300 mg cohort. The predefined criteria for success were met for the 400 mg regimen but not for the 300 mg regimen.
These promising results served as the ‘precursor’ for PROfound, a randomized, open-label, phase III trial of olaparib 300 mg twice daily versus physician’s choice of standard of care therapy in men with HRR-mutated mCRPC who had disease progression while receiving a novel hormonal agent (e.g., enzalutamide or abiraterone). Patients were assigned to one of two cohorts based on their HRR gene alteration. Cohort A included patients with BRCA1, BRCA2, or ATM alterations, irrespective of co-occurring alterations in any other HRR genes. Cohort B had patients with alterations in any of the other 12 HRR genes (BRIP1, BARD1, CDK12, CHEK 1/2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, RAD54L). Patients within each cohort were randomized in 2:1 fashion to olaparib versus standard of care . The primary endpoint was the rPFS in cohort A.
Of the 4,425 enrolled patients, 4,047 had tumor tissue available for testing and only 2,792 (69%) were successfully sequenced. A qualifying alteration in one or more of the 15 HRR genes was detected in 778 of 2,792 patients (28%). Median rPFS was significantly longer in the olaparib group than in the standard of care group (7.4 months versus 3.6 months; HR: 0.34; 95% CI: 0.25 – 0.47; p<0.001).
The confirmed objective response rate (ORR) was 33% in the olaparib group and 2% in the standard of care group (odds ratio 20.9; 95% CI: 4.2 – 379.2; p<0.001). The median time to pain progression was also significantly longer in the olaparib group (HR: 0.44; 95% CI: 0.22 – 0.91; p=0.02). The final overall survival (OS) analysis demonstrated that olaparib improved OS in cohort A from a median of 14.7 to 19.1 months (HR: 0.69, 95% CI: 0.50 – 0.97). Notably, 84% of patients with imaging-based disease progression had crossed over from the standard of care arm to olaparib at the time of analysis, which highlights the efficacy of earlier use of olaparib in this setting.10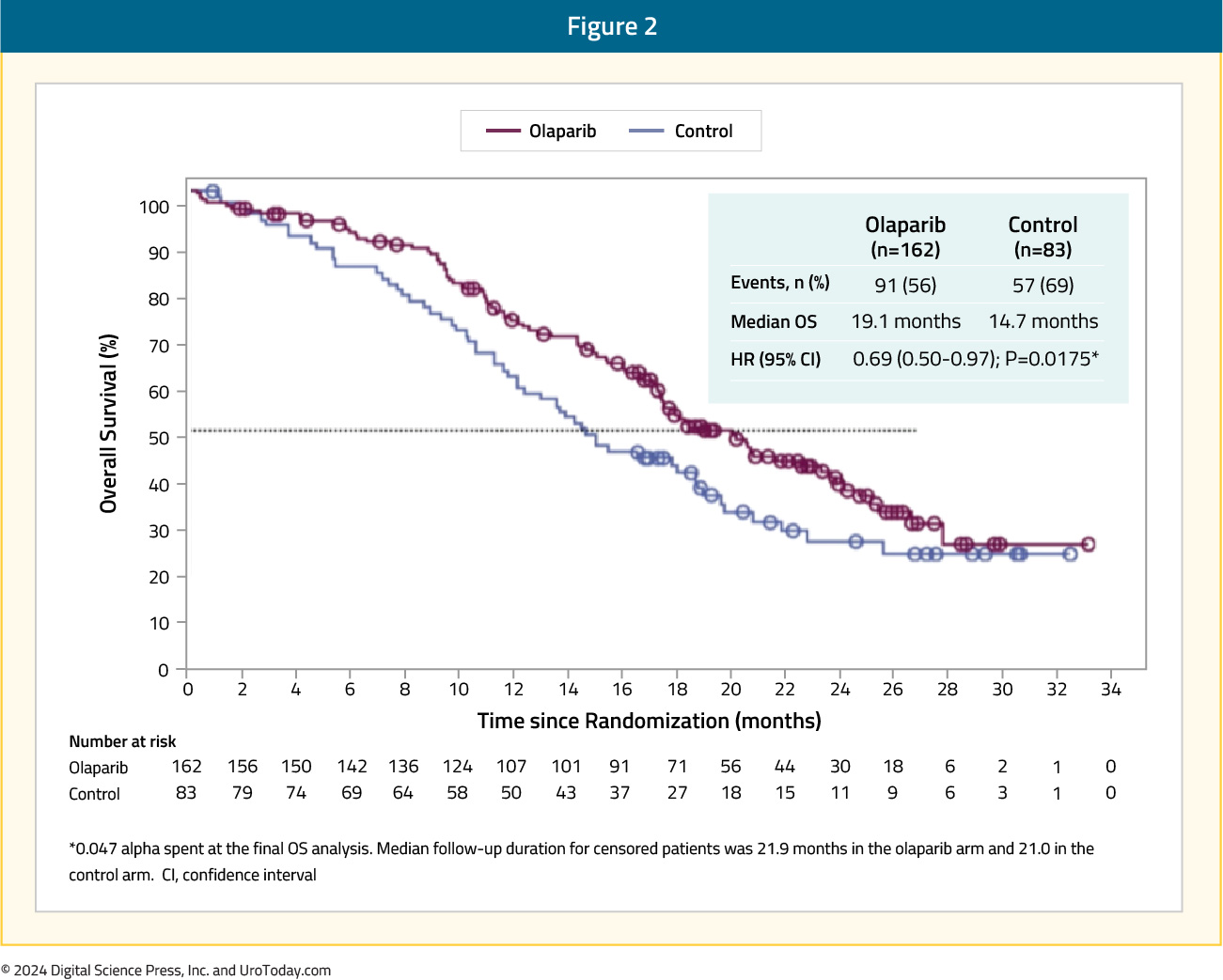
The data from PROfound formed the basis for the FDA-approval of olaparib 300 mg PO twice daily in men with HRR-mutated mCRPC after progression on enzalutamide or abiraterone.4
Rucaparib
The first PARP inhibitor to be approved by the FDA for the treatment of prostate cancer patients was rucaparib. On May 15, 2020, rucaparib was granted accelerated approval for patients with mCRPC and BRCA mutations (germline or somatic) who had progressed following treatment with androgen receptor-directed therapy and a taxane-based chemotherapy.3 This approval was based on the results of TRITON2, which was initially published in 202011 and most recently updated in 2023.12 TRITON2 is an international, open-label, phase II trial that evaluated the safety and efficacy of rucaparib 600 mg twice daily in mCRPC patients with DNA damage response (DDR) gene alterations who had progressed after 1–2 lines of an androgen receptor pathway inhibitor and one taxane-based chemotherapy. The efficacy cohort included 277 patients, of whom 172 (62.1%) had a deleterious germline or somatic BRCA alteration with 21.3%, 5.4%, 3.1%, 4%, and 4.7% having ATM, CDK12, CHEK2, PALB2, and other DDR gene mutations, respectively. A confirmed objective response was observed in 46% of BRCA patients with measurable disease (10% complete response). A superior response was observed among BRCA2 patients (48% versus 30% for BRCA1), which is potentially secondary to an increased frequency of biallelic mutations among BRCA2 patients and a greater coexistence of TP53 mutations among BRCA1-mutated men.13 The objective response was consistent irrespective of whether the BRCA mutation was somatic or germline and whether other DDR mutations were present or absent. All four patients with PALB2 mutations and measurable disease had an objective partial response, with none of the ATM-, CDK12-, CHEK2-mutated patients experiencing an objective response. A confirmed PSA50 response was observed in 53% and 55% of BRCA and PALB2-mutated patients, compared to 3.4–14% among patients with other DDR gene mutations. The median overall survival was 17.2 months for BRCA patients, compared to 11.1–14.6 months among ATM, CDK12, and CHEK2-mutated patients.
Following the promising results of TRITON2, the phase 3 TRITON3 trial was published in 2023. This was a randomized phase 3 trial of mCRPC patients with a BRCA1, BRCA2, or ATM alterations who experienced disease progression following treatment with a second-generation androgen receptor pathway inhibitor. Patients underwent 2:1 randomization to receive oral rucaparib (600 mg twice daily) or a physician’s choice control (docetaxel or a second-generation ARPI [abiraterone acetate or enzalutamide]). The primary outcome was the median PFS according to independent review. There were 405 patients randomized to receive rucaparib (n=270) or the control group (n=135). At 62 months follow-up, imaging-based PFS was significantly prolonged in the rucaparib group compared to the control group, both in the BRCA subgroup (11.2 and 6.4 months, respectively; HR: 0.50; 95% CI: 0.36 – 0.69) and in the intention-to-treat population (10.2 and 6.4 months, respectively; HR: 0.61; 95% CI: 0.47 – 0.80; p<0.001 for both comparisons). No significant PFS benefit was observed in the ATM subgroup.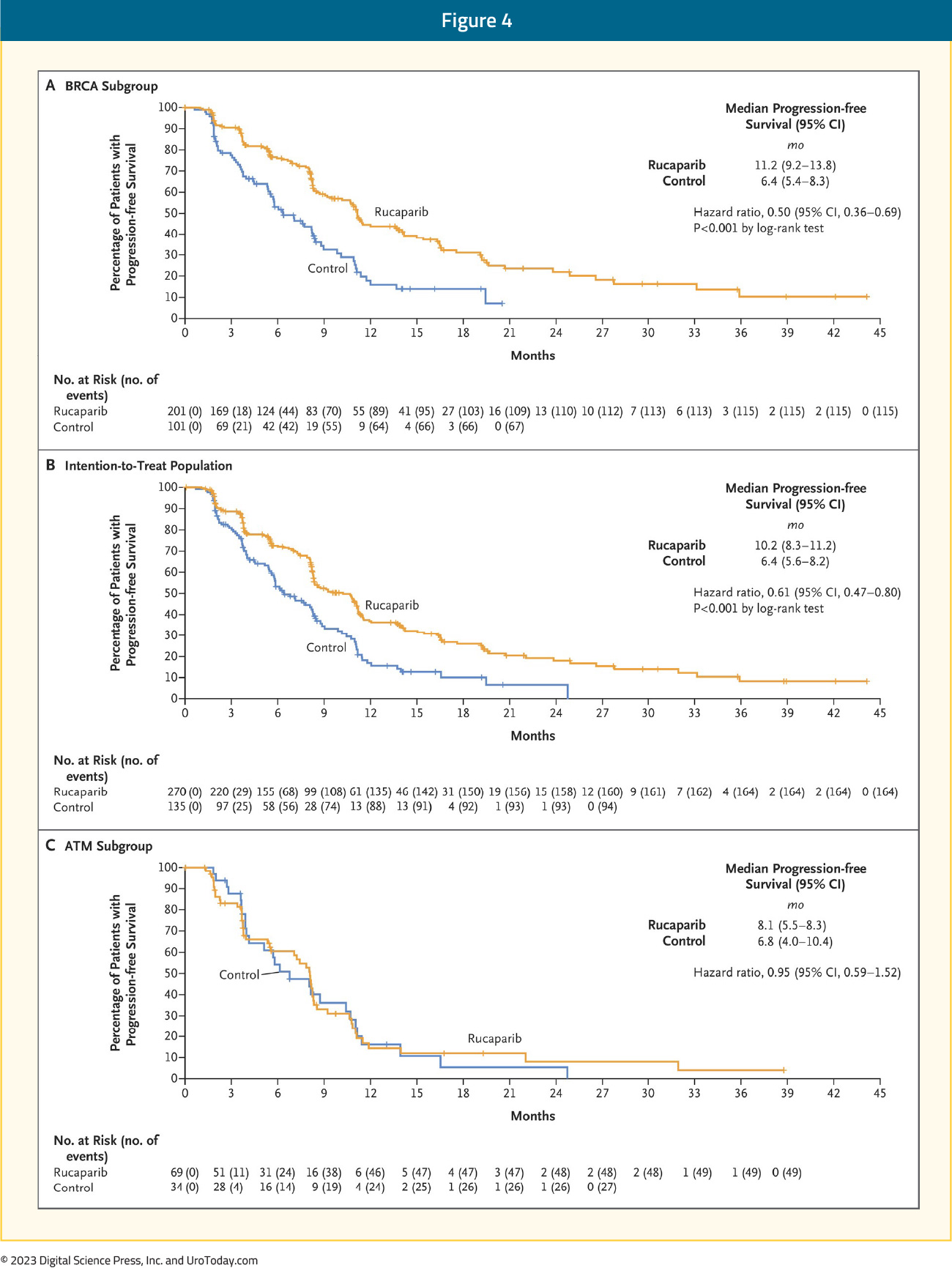
In the BRCA subgroup, the median OS was 24.4 versus 20.8 months in favor of rucaparib (HR: 0.81, 95% CI: 0.58 – 1.12, p=0.21).14
Talazoparib
TALAPRO-1 was an open-label, phase II trial that evaluated talazoparib 1 mg/day in patients with evidence of progressive mCRPC who had measurable soft-tissue disease and evidence of one of 11 DDR mutations who had progressed following taxane-based chemotherapy (48% both docetaxel and cabazitaxel) and abiraterone and/or enzalutamide (98% of population). The primary endpoint was confirmed ORR. There were 128 patients enrolled, of whom 127 received at least one dose of talazoparib (safety population) and 104 had measurable soft-tissue disease (antitumor activity population). After a median follow-up of 16.4 months, the ORR was 30% (95% CI: 21.2 – 39.6%).15
Niraparib
GALAHAD was a multicenter, open-label, single arm phase II trial of 289 mCRPC patients with DNA repair gene defects and disease progression following a prior next-generation androgen signaling inhibitor and a taxane, who received niraparib 300 mg orally once daily. The primary endpoint was ORR in patients with BRCA alterations and measurable disease. At a median follow-up of 10 months, the ORR in the measurable BRCA cohort was 34.2%. The median duration of objective response was 5.6 months. Conversely, the ORR in the measurable non-BRCA cohort was 10.6%. Median rPFS (8.1 versus 3.7 months) and OS (13.0 and 9.6 months) were both longer in the BRCA cohort, compared to the non-BRCA cohort.16
Management of Side Effects of PARP Inhibitors
The adverse event/safety profiles of all PARP inhibitors overlap considerably. The most common (any CTCAE grade) clinical side effects in phase III trials of rucaparib, olaparib and niraparib include:17
- Nausea: ~75%
- Fatigue: 60–70%
- Vomiting: ~35%
- Constipation: 20–40%
- Dysgeusia: 10–40%
- Anorexia: ~25%
- Abdominal pain: 25–30%
- Diarrhea: 20–30%
- Headache: 20–25%
- Cough: 10–15%
The most common (any CTCAE grade) lab abnormalities were:
- Anemia: 40–50%
- Thrombocytopenia: 15–60%
- Neutropenia: 20–30%
- Alanine aminotransferase (ALT) elevation: 5–36%
- Aspartate aminotransferase (AST) elevation: 2–28%
- Increased serum creatinine level: 10–15%
While nausea is the most common side effect of PARP inhibitor therapy, it tends to be mild in most cases. This side effect can be managed by taking the medication after a meal and an antiemetic (prochlorperazine or a 5-HT3 antagonist such as ondansetron) may be considered in patients who develop moderate or severe nausea and/or vomiting with PARP inhibitor therapy.
Close monitoring of patients following PARP inhibitor therapy initiation is required, particularly in the first three months, as hematologic adverse effects usually occur early, but not invariably, and regular blood counts should continue while patients are on treatment. Anemia is the most common hematologic toxicity observed with PARP inhibitors, with grade 3–4 anemia observed in 22% of patients on olaparib, 27% of patients on rucaparib, and 31% of patients on niraparib first-line maintenance therapy ovarian cancer trials.18-21 The management of such events may include dose reductions and/or interruptions, with transfusions reserved for symptomatic anemic events or if the hemoglobin level falls to <7 g/dL. Thrombocytopenia appears to be more common with niraparib at 61%, as opposed to olaparib (14%) or rucaparib (28%). The niraparib FDA label thus recommends obtaining weekly platelet levels during the first month of therapy.
Elevated serum creatinine level occurs within the first few weeks of therapy and is thought to be an on-target effect due to the inhibition of renal transporter proteins. Thus, serum creatinine-based estimation of renal function may be inaccurate in patients receiving PARP inhibitor therapy. Alternative methods of glomerular filtration rate (GFR) estimation such as radionuclide scan or serum-cystatin C must be used in cases where a more accurate GFR estimate is necessary. Elevation of AST and ALT also tends to typically occur within the first two cycles and can be transient. Treatment interruption may not be required for mild AST/ALT elevations, but serum bilirubin levels must be checked in all patients to evaluate for drug-induced liver injury.
Owing to their mechanism of action, there was a concern regarding treatment-emergent myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) with PARP inhibitor therapies. However, it appears that the risk of MDS/AML is <1.5%. Of the 2,351 patients treated in olaparib monotherapy trials, only 28 (<1.5%) developed MDS/AML. Of these, 25/28 patients had a BRCA mutation, two patients had a wild-type germline BRCA, and one patient had unknown BRCA mutation status. The duration of olaparib varied from < 6 months to > 2 years and all had received previous chemotherapy with platinum and/or other DNA damaging agents, or radiotherapy.17 If pancytopenia occurs at any point during PARP inhibitor therapy, treatment must be interrupted as per guidelines for the drug, and appropriate evaluation for MDS and AML must be undertaken. Therapy must be discontinued permanently if a diagnosis of MDS or AML is confirmed.
Another important consideration is the potential for clinically-significant drug-drug interactions (DDI) with all PARP inhibitors. Rucaparib and olaparib are primarily metabolized by different members of the cytochrome P450 enzyme family, resulting in only a partial overlap in DDIs. Niraparib is metabolized in the liver by carboxylesterase-catalyzed amide hydrolysis with cytochrome P450 playing only a negligible role.22 Many commonly used drugs (such as phenytoin, carbamazepine, ketoconazole, ciprofloxacin, digoxin) have uni- or bi-directional interactions with PARP inhibitors. Thus, careful attention must be paid to minimize DDI by avoiding, discontinuing, adjusting the dose, or clinical/lab monitoring of these medications before and during PARP therapy. Involving a dedicated oncology pharmacist, where available, may be a valuable aid in this treatment setting.
Conclusions and Future Directions
PARP inhibitor monotherapy has demonstrated promising outcomes for the treatment of HRR-mutated mCRPC patients with evidence of disease progression following treatment with an androgen receptor pathway inhibitor and/or taxane-based chemotherapy. As a result, there has been an increased interest in ‘moving up’ these agents along the disease spectrum, as well as combining PARP inhibitors with other agents that may have a synergistic mechanism of action.
Published March 2024
- Written by: Rashid K. Sayyid, MD, MSc Urologic Oncology Fellow University of Toronto Toronto, ON and Zachary Klaassen, MD, MSc Associate Professor Wellstar MCG Health Augusta, GA
- References:
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 2016;375:443-453.
- Xie T, Dickson K, Yee C, et al. Targeting Homologous Recombination Deficiency in Ovarian Cancer with PARP Inhibitors: Synthetic Lethal Strategies That Impact Overall Survival. Cancers (Basel). 2022;14(19):4621.
- FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate. Accessed on March 8, 2024.
- FDA approves olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer. Accessed on March 8, 2024.
- FDA approves olaparib with abiraterone and prednisone (or prednisolone) for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-abiraterone-and-prednisone-or-prednisolone-brca-mutated-metastatic-castration. Accessed on March 8, 2024.
- FDA approves niraparib and abiraterone acetate plus prednisone for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-and-abiraterone-acetate-plus-prednisone-brca-mutated-metastatic-castration. Accessed on March 8, 2024.
- FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate. Accessed on March 8, 2024.
- Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-1708.
- Mateo J, Porta N, Bianchini D, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):162-174.
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;383:2345-2357.
- Abida W, Campbell D, Patnaik A, et al. Non-BRCA DNA Damage Repair Gene Alterations and Response to the PARP Inhibitor Rucaparib in Metastatic Castration-Resistant Prostate Cancer: Analysis From the Phase II TRITON2 Study. Clin Cancer Res. 2020;26(11):2487-2496.
- Abida W, Campbell D, Patnaik A, et al. Rucaparib for the Treatment of Metastatic Castration-resistant Prostate Cancer Associated with a DNA Damage Repair Gene Alteration: Final Results from the Phase 2 TRITON2 Study. Eur Urol 2023;84:321-330.
- Taza F, Holler AE, Fu W, et al. Differential Activity of PARP Inhibitors in BRCA1- Versus BRCA2-Altered Metastatic Castration-Resistant Prostate Cancer. JCO Precis Oncol 2021;5:PO.21.00070.
- Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N Engl J Med. 2023;388:719-732.
- de Bono JS, Mehra N, Scagliotti GV, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol. 2021;22(9):1250-1264.
- Smith MR, Scher HI, Sandhu S, et al. Niraparib in patients with metastatic castration-resistant prostate cancer and DNA repair gene defects (GALAHAD): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2022;23(3):362-373.
- LaFargue C, Dal Molin DZ, Sood AK, et al. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20(1):e15-e28.
- Gonzalez-Martin A, Pothuri B, Vergote I, et al: Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391-2402.
- Moore K, Colombo N, Scambia G, et al: Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Banerjee S, Moore KN, Colombo N, et al: Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-Year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
- Monk BJ, Parkinson C, Lim MC, et al: A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964.
- Sandhu SK, Schelman WR, Wilding G, et al. The poly (ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;13(9):882-892.
Racial Disparities in Bladder Cancer
The Quickly Evolving Treatment Landscape of Metastatic Urothelial Carcinoma: A New Standard of Care in First-Line Systemic Therapy
Introduction
Metastatic urothelial carcinoma is associated with a poor prognosis, with an estimated 17,000 deaths annually in the United States from this disease.1 Platinum-based chemotherapy had long been considered the standard of care first line treatment for platinum-eligible patients with metastatic urothelial carcinoma.- Written by: Zachary Klaassen, MD, MSc Associate Professor of Urology Urologic Oncologist Medical College of Georgia, Georgia Cancer Center Augusta, GA and Rashid Sayyid, MD, MSc Urologic Oncology Fellow University of Toronto Toronto, Ontario, Canada
- References:
- American Cancer Society. Key Statistics for Bladder Cancer. Accessed on February 26, 2024.
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18: 3068-3077.
- Sternberg CN, de Mulder PH, Schornagel JH, et al. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol. 2001;19: 2638-2646.
- Powles T, Csoszi T, Ozguroglu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(7): 931-945.
- Galsky MD, Arija JAA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236): 1547-1557.
- Powles TB, Perez Calderrama B, Gupta S, et al. LBA6 EV-302/KEYNOTE-A39: Open-label, randomized phase III study of enfortumab vedotin in combination with pembrolizumab (EV+P) vs chemotherapy (Chemo) in previously untreated locally advanced metastatic urothelial carcinoma (la/mUC). Annal Oncol. 2023;34(Suppl 2): S1340.
- van der Heijden MS, Sonpavde G, Powles T, et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023;389(19): 1778-1779.
- FDA approves enfortumab vedotin-ejfv with pembrolizumab for locally advanced or metastatic urothelial cancer. Accessed on February 26, 2024.
- U.S. Food and Drug Administration Accepts for Priority Review Bristol Myers Squibb’s Application for Opdivo (nivolumab) in Combination with Cisplatin-Based Chemotherapy for the First-Line Treatment of Adult Patients with Unresectable or Metastatic Urothelial Carcinoma. Accessed on February 26, 2024.U.S. Food and Drug Administration Accepts for Priority Review Bristol Myers Squibb’s Application for Opdivo (nivolumab) in Combination with Cisplatin-Based Chemotherapy for the First-Line Treatment of Adult Patients with Unresectable or Metastatic Urothelial Carcinoma. Accessed on February 26, 2024.
- Klumper N, Ralser DJ, Ellinger J, et al. Membranous NECTIN-4 Expression Frequently Decreases during Metastatic Spread of Urothelial Carcinoma and Is Associated with Enfortumab Vedotin Resistance. Clin Cancer Res. 2023;29(8): 1496-1505.
- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med. 2021;384: 1125-35.
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376(11): 1015-1026.
- Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer "unfit" for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29: 2432-2438.
- Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol. 1985;133: 403-407.
- Sternberg CN, Yagoda A, Scher HI, et al. Methotrexate, vinblastine, doxorubicin, and cisplatin for advanced transitional cell carcinoma of the urothelium. Efficacy and patterns of response and relapse. Cancer. 1989;64: 2448-2458.
- Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17: 3173-3181.
- Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42: 50-54.
- Lee YS, Ha MS, Tae JH, et al. Gemcitabine-cisplatin versus MVAC chemotherapy for urothelial carcinoma: a nationwide cohort study. Sci Rep. 2023;13: 3682.
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020;383: 1218-1230.
- Powles T, Park SH, Caserta C, et al. Avelumab First-Line Maintenance for Advanced Urothelial Carcinoma: Results From the JAVELIN Bladder 100 Trial After ≥2 Years of Follow-Up. J Clin Oncol. 2023;41: 3486-3492.
- Powles T, Sridhar SS, Loriot Y, et al. Avelumab maintenance in advanced urothelial carcinoma: biomarker analysis of the phase 3 JAVELIN Bladder 100 trial. Nat Med. 2021;27: 2200-2211
Radioligand Therapy for Metastatic Castrate-Resistant Prostate Cancer: Radium-223, 177Lu-PSMA-617, New Agents, and Novel Combinations
Introduction
The treatment landscape of metastatic castrate-resistant prostate cancer (mCRPC) has long been dominated by androgen receptor pathway inhibitors (ARPIs) and chemotherapeutic agents. There are currently, however, two radioligands that are United States Food and Drug Administration (FDA)-approved for the treatment of mCRPC patients:
- Written by: Rashid K. Sayyid, MD, MSc, University of Southern California, Los Angeles, CA and Zachary Klaassen, MD, MSc, Wellstar MCG Health, Augusta, GA
- References:
- FDA Approves Radium-223 for Advanced Prostate Cancer. https://www.fda.gov/drugsatfda. Access on Aug 5, 2024.
- FDA approves Pluvicto for metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer. Accessed on Aug 5, 2024.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-23.
- Higano CS, George DJ, Shore ND, et al. Clinical outcomes and treatment patterns in REASSURE: planned interim analysis of a real-world observational study of radium-223 in metastatic castration-resistant prostate cancer. EClinicalMedicine. 2023;60:101993.
- Raval AD, Zhang Y, Korn MJ, et al. Real-world utilization patterns of radium-223 in metastatic prostate cancer in the United States: An administrative claims database study. J Clin Oncol. 2024;42:Number 4_suppl.
- Herranz UA, Calvo OF, Breijo SM, et al. Radium-223 for mCRPC, a real-world experience study from 7 Galician medical centers. J Clin Oncol. 2023;41:Number 6_suppl.
- Chang SS. Overview of Prostate-Specific Membrane Antigen. Rev Urol. 2004;6(Suppl 10):S13-S18.
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1091-103.
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- Hofman MS, Emmett L, Sandhu S, et al. Overall survival with [177Lu]Lu-PSMA-617 versus cabazitaxel in metastatic castration-resistant prostate cancer (TheraP): secondary outcomes of a randomised, open-label, phase 2 trial. Lancet Oncol. 2024;25(1):99-107.
- Rahbar K, Essler M, Pabst KM, et al. Safety and Survival Outcomes of 177Lu-Prostate-Specific Membrane Antigen Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer with Prior 223Ra treatment: The RALU Study. J Nucl Med. 2023;64(4):574-8.
- Heskmap S, Hernandez R, Molkenboer-Kuenen JDM, et al. α- Versus β-Emitting Radionuclides for Pretargeted Radioimmunotherapy of Carcinoembryonic Antigen–Expressing Human Colon Cancer Xenografts. J Nucl Med. 2017;58(6):926-33.
- Sathekge MM, Lawal IO, Bal C, et al. Actinium-225-PSMA radioligand therapy of metastatic castration-resistant prostate cancer (WARMTH Act): a multicentre, retrospective study. Lancet Oncol. 2024;25(2):175-83.
- Emmett L, Subramaniam S, Crumbaker M, et al. [177Lu]Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer (ENZA-p): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 2024;25(5): 563-71.
- Pan E, Xie W, Ajmera A, et al. A Phase I Study of Combination Olaparib and Radium-223 in Men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) with Bone Metastases (COMRADE). Mol Cancer Ther. 2023;22(4):511-8.
- Scholz A, Knuuttila M, Zdrojewska J, et al. Abstract 5043: Radium-223 demonstrates increased antitumor activity in combination with 177Lu-PSMA-617 in the intratibial LNCaP xenograft model of bone metastatic prostate cancer. Cancer Res. 2023;93(7_Supplement):5043.
Lessons Learned from COVID-19 in the Care of NMIBC Patients: Insights from the CISTO Investigators
Gem/Doce in 2024: What is Next?
- Written by: Vignesh Packiam, MD, Associate Professor, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ


