Introduction
Radium-223 dichloride (radium-223) is a targeted alpha emitter that selectively binds to areas of increased bone turnover in bone metastases and emits high-energy alpha particles of short-range (<100 μm). Radium-223 acts as a bone-seeking calcium mimetic and binds into newly formed bone stroma, especially within the microenvironment of osteoblastic or sclerotic metastases. Double-stranded DNA breaks result secondary to the high-energy alpha-particle radiation. This results in a potent and highly localized cytotoxic effect in the target areas. Furthermore, the short path of the alpha particles also theoretically minimizes the toxic effects on adjacent healthy tissue, including the bone marrow.Radium-223 has been approved in mCRPC patients for more than a decade based on the overall survival benefit noted in the ALSYMPCA trial published in 2013.1 However, radium-223 utilization, particularly over the last several years, has decreased for a variety of reasons.2 The 2024 ESMO presentation of the PEACE-3 trial assessing radium-223 + enzalutamide versus enzalutamide alone in first-line mCRPC may resurrect radium-223 back to the forefront of mCRPC therapy. This Center of Excellence article will review the pivotal ALSYMPCA trial that led to initial FDA approval of radium-223, explore several hypotheses as to why radium-223 utilization has decreased in recent years, highlight the recent PEACE-3 trial findings, and emphasize implications and patient selection for utilizing radium-223 + enzalutamide in first-line mCRPC.
The ALSYMPCA Trial
The ALSYMPCA trial was a phase III, randomized, double-blind, placebo-controlled trial that randomized 921 patients in a 2:1 fashion to receive six injections of radium-223 (at a dose of 50 kBq/kg intravenously) or matching placebo. All patients received additional best standard of care and were required to have symptomatic disease with regular use of analgesic medication or treatment with external beam radiotherapy for cancer-related bone pain within the preceding 12 weeks. Of note, patients could have received prior docetaxel (57% of included patients). The trial design of ALSYMPCA is as follows: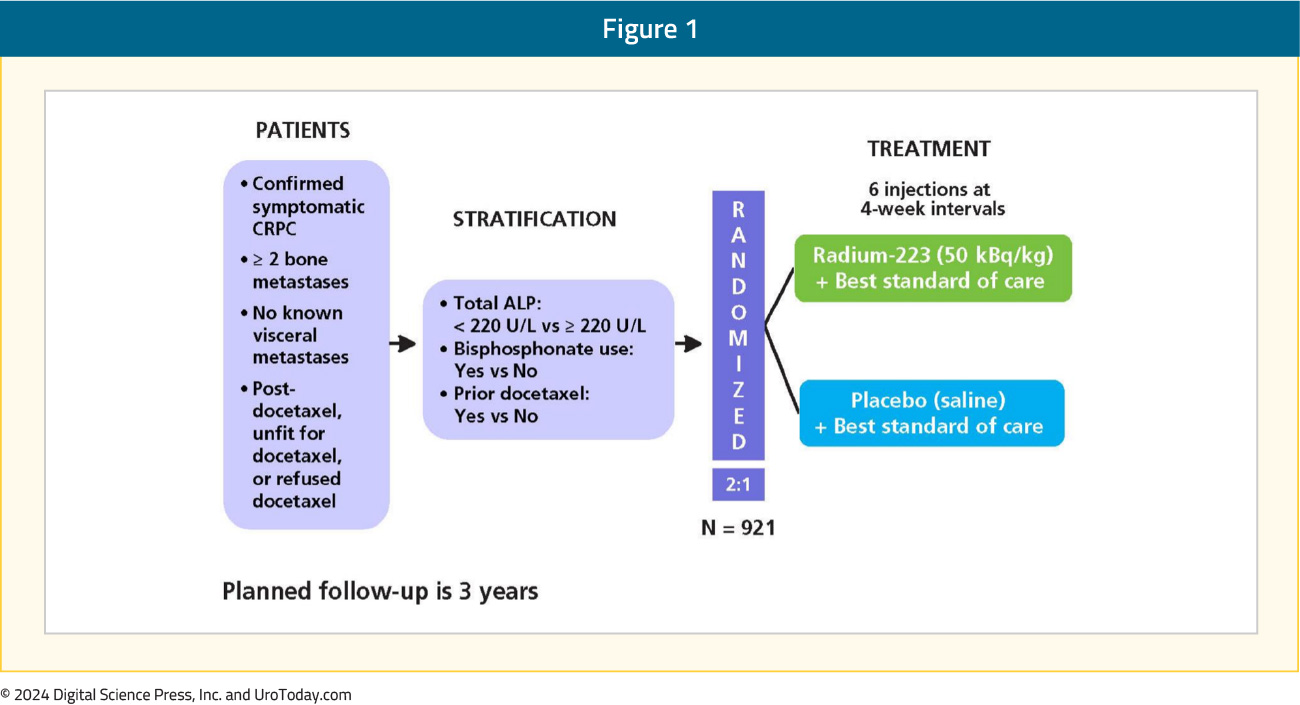
Patients receiving radium-223 had significantly improved median overall survival (14.9 months) versus placebo (11.3 months; HR 0.70, 95% CI 0.58–0.83):
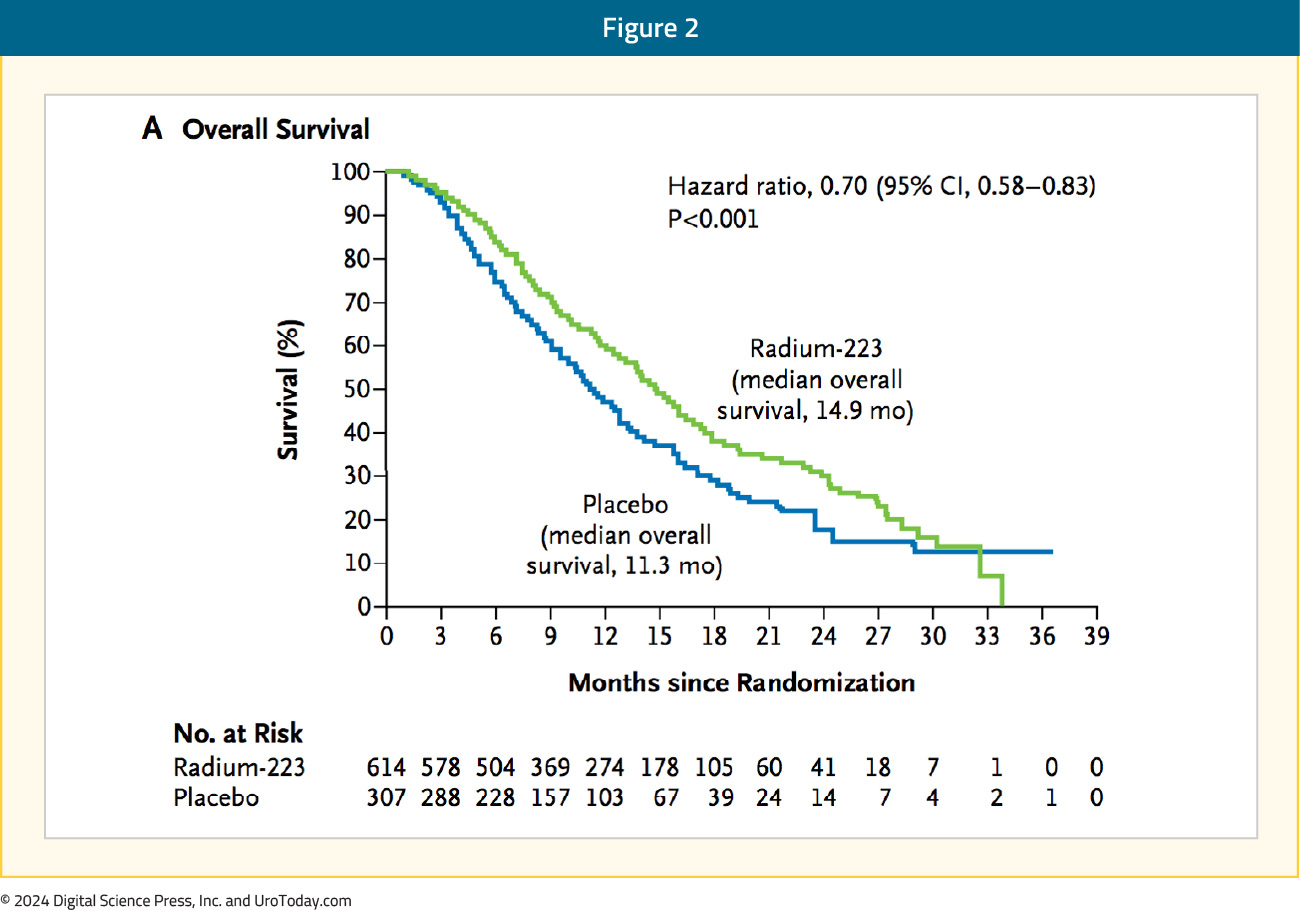
Use of radium-223 was also associated with significantly prolonged time to the first symptomatic skeletal event (15.6 versus 9.8 months, HR 0.66, 95% CI 0.52–0.83):
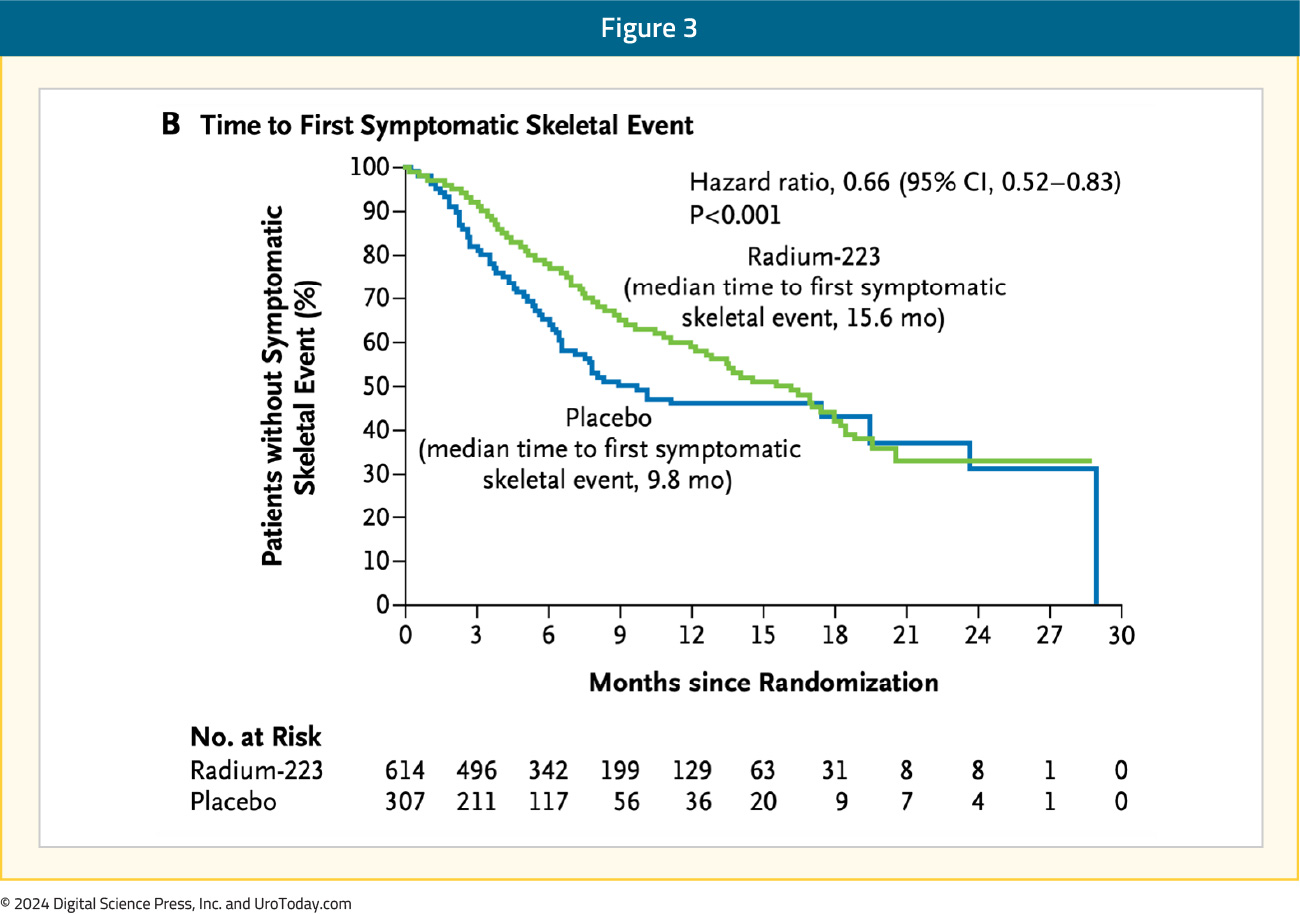
Moreover, radium-223 significantly prolonged the time to increase in total alkaline phosphatase level (HR 0.17, 95% CI 0.13-0.22) and time to increase in PSA level (HR 0.64, 0.54–0.77). Grade 3-4 adverse events were seen less frequently in the treatment arm (56% versus 62%). These results led to the FDA approval of radium-223; however, this approval was limited by the reliance on the calcium mimetic mechanism of action. As a result, it is only suitable for and approved for patients with symptomatic bone-only disease.
Underutilization of Radium-223
Since the approval of radium-223 in 2013, its utilization and uptake in clinical practice have arguably been less robust than expected. Previous studies have suggested that radium-223 may be utilized as a later agent and is often one of the last treatments patients receive.2 Dr. Sartor has previously discussed possible reasons for poor utilization, with the following hypotheses and thoughts regarding improved patient selection:
A drastic change in the treatment landscape: Options for therapy when patients were accruing to ALSYMPCA (2008-2011) were far fewer, compared to the plethora of treatment options currently available.
Radium-223 targets activated bone stromal lesions, but not soft tissue tumors: Better patient selection is needed for radium-223. Positive selection criteria include bone scans, and possibly F-18 NaF PET uptake, which binds avidly and quantitatively to bone stroma. Negative selection criteria are PSMA PET positive visceral and soft tissue lesions, which has greater sensitivity compared to CT or MRI scans. Ahmadzadehfar and colleagues previously looked at 68Ga-PSMA-11 PET as a gatekeeper for the treatment of metastatic prostate cancer with radium-223.3 As can be seen in the following PSA waterfall plots, patient selection with bone scan and PSMA PET/CT is a move in the right direction, compared to with bone scan and CT scan only. Patient selection with bone and CT scan only:
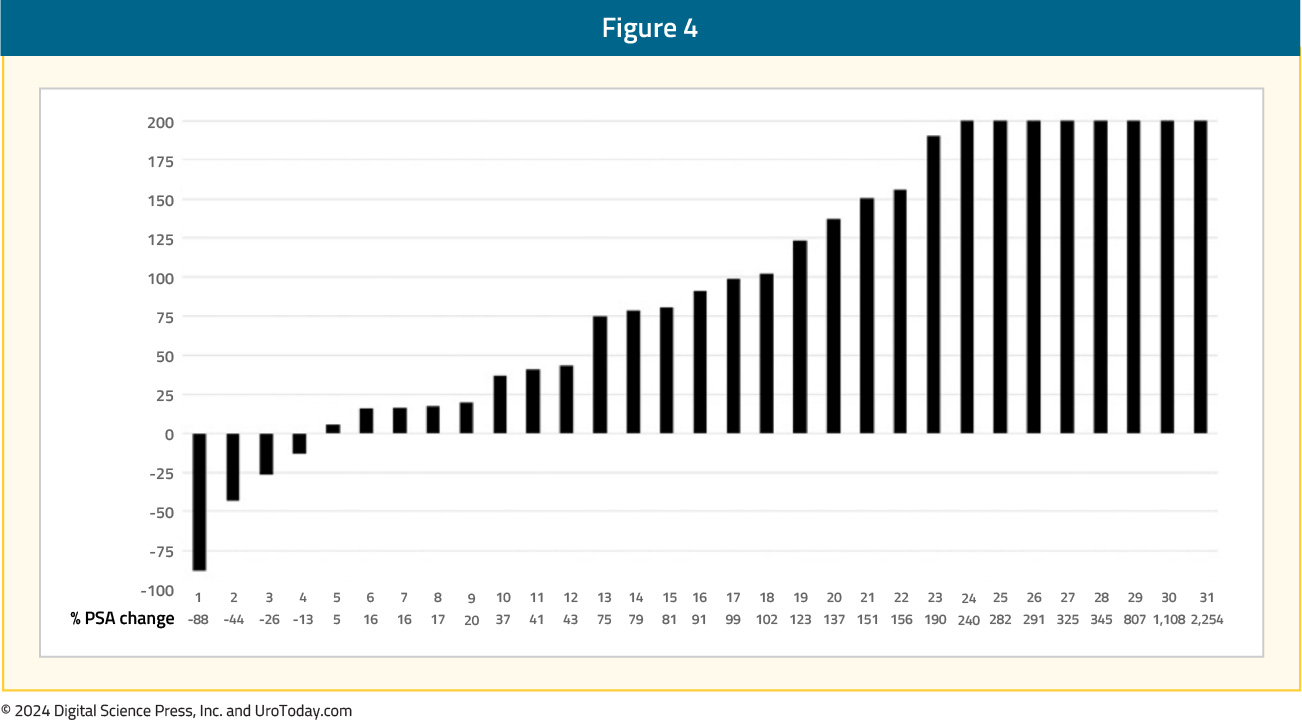
Patient selection with bone scan and PSMA PET/CT:
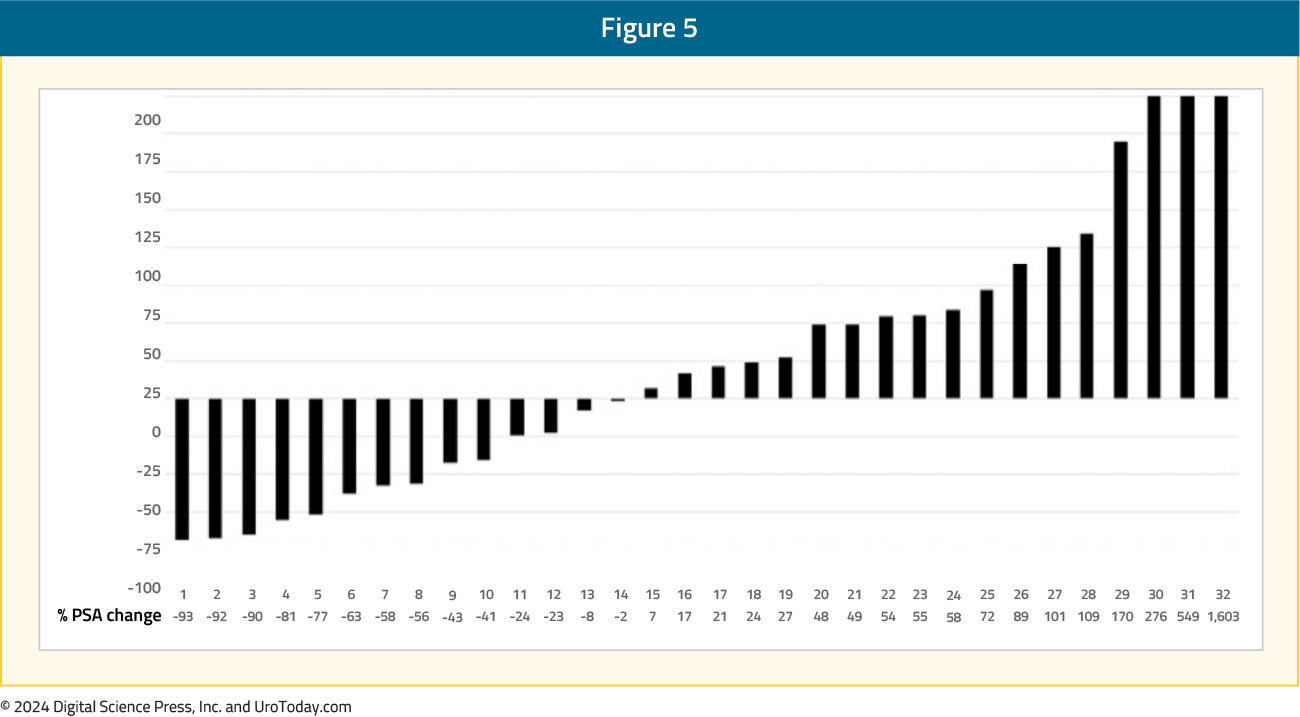
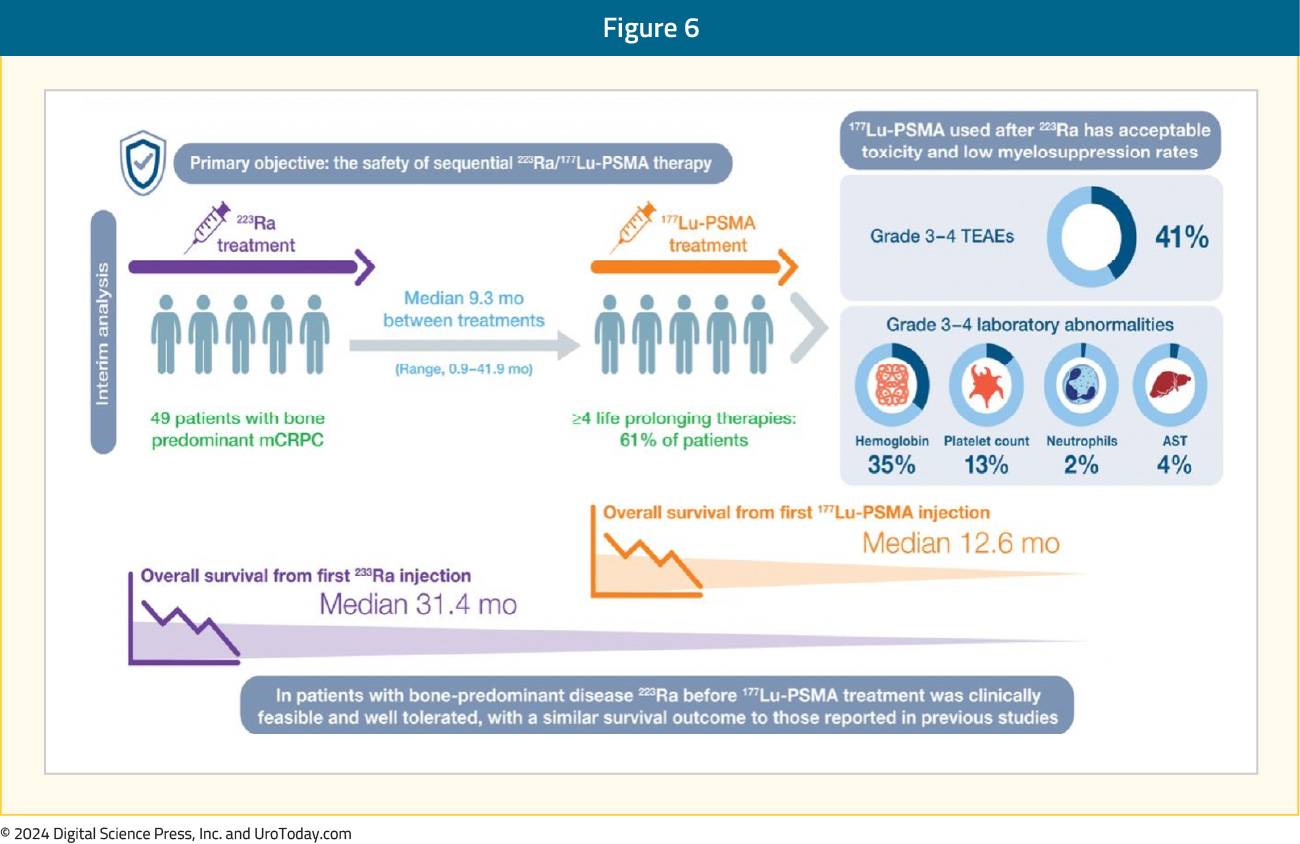
PSMA PET and diffusion-weighted MRI provide better response monitoring after radium-223 treatment: The interim analysis of the RALU study,4 published in 2023, showed that the median time between treatment with radium-223 and 177Lu-PSMA therapy was 9.3 months (range: 0.9–41.9), with acceptable toxicity given the grade 3-4 treatment-emergent adverse event rate was 41%. The median overall survival from the first radium-223 injection was 31.4 months and the overall survival from the first 177Lu-PSMA injection was 12.6 months. Thus, in patients with bone-predominant disease, radium-223 before 177Lu-PSMA treatment was clinically feasible and well tolerated, with a similar survival outcome to those reported in previous studies:
The PEACE-3 Trial
Abiraterone5 and enzalutamide6 are standard-of-care options for first-line treatment of patients with mCRPC that have progressed on ADT. However, no combination thus far has been proven to increase both radiographic progression-free survival and overall survival in the first-line mCRPC treatment setting. Thus, there is biologic rationale for combining additional agents with unique mechanisms of action for first-line mCRPC therapy.EORTC-GUCG 1333 (PEACE-3) is a prospective phase III trial of mCRPC patients with bone metastases who were asymptomatic or mildly symptomatic. Eligible patients had not received prior enzalutamide or radium-223 and had no known visceral metastases; prior treatment with abiraterone and docetaxel in the hormone-sensitive setting was permissible. Study participants underwent 1:1 randomization to radium-223 55 kBq/kg IV every 4 weeks for 6 cycles + enzalutamide 160 mg orally once daily versus enzalutamide 160 mg orally once daily. The primary endpoint was radiographic progression-free survival. Key secondary endpoints included: (i) safety, (ii) overall survival, (iii) time to next treatment, (iv) time to pain progression, and (v) time to first symptomatic skeletal event. Given the increased rate of skeletal fractures reported in the ERA-223 trial, a study amendment was introduced following the enrollment of the initial 119 patients to mandate the use of bone protective agents. The trial design for PEACE-3 is as follows:7
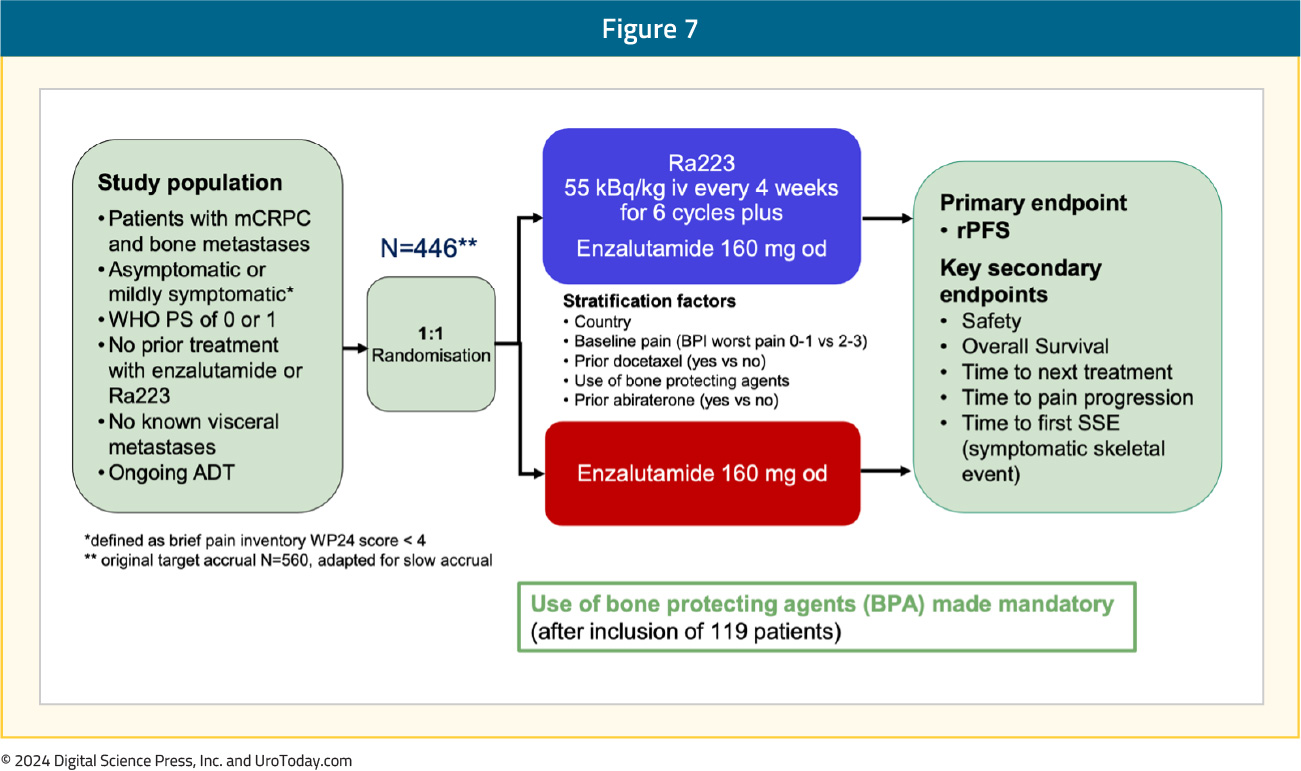
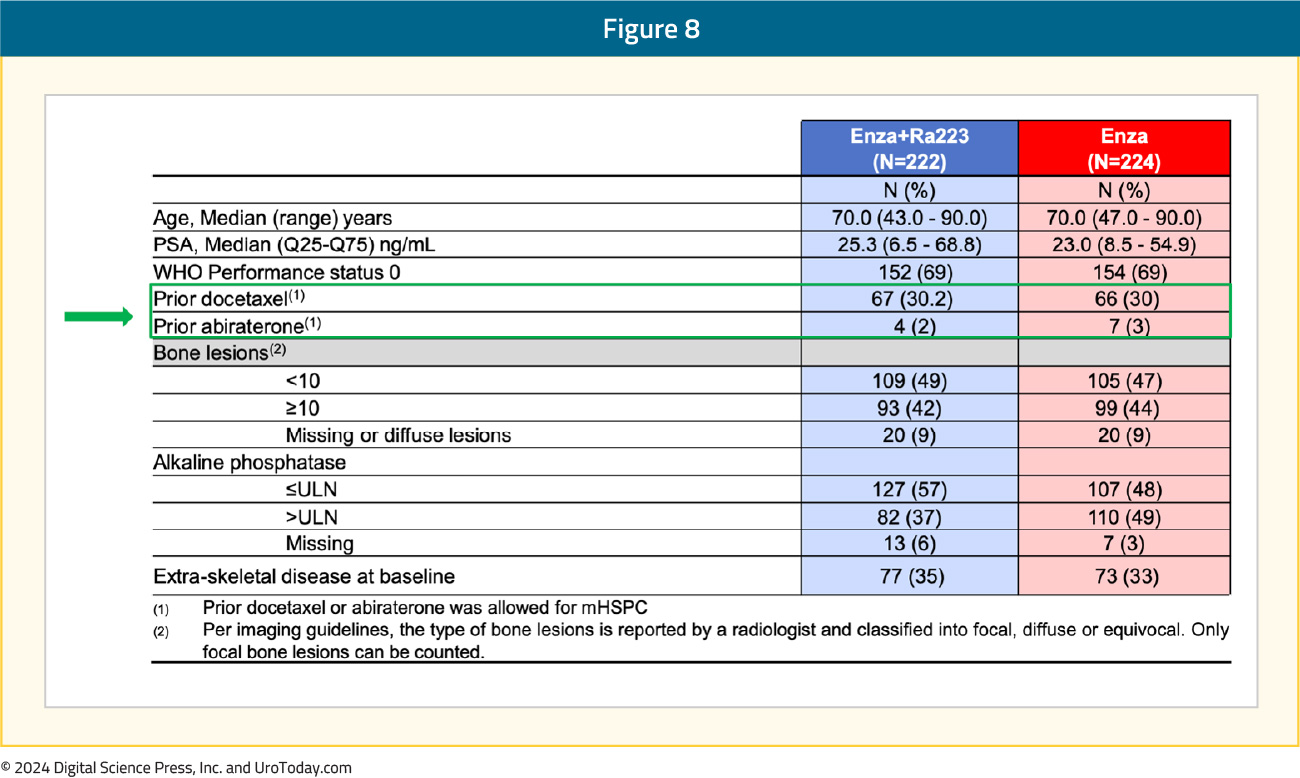
Between November 2015 and March 2023, 446 patients were enrolled from 12 countries. The median study follow-up was 42.2 months. The median patient age was 70, the median PSA was 23–25 ng/ml, approximately 30% of patients had received prior docetaxel in the hormone-sensitive setting, and only 2–3% had received prior abiraterone. The baseline patient characteristics are summarized below:
Of the 222 patients in the radium-223 + enzalutamide arm, 215 (97%) received both treatments and of the patients who received radium-223, 88% completed all 6 planned cycles. The addition of radium-223 to enzalutamide was associated with a significant improvement in radiographic progression-free survival (median: 19.4 versus 16.4 months; HR 0.69, 95% CI 0.54–0.87). At 24 months, 45% of patients in the radium-223 combination arm were free of radiographic progression, compared to 36% of patients in the enzalutamide monotherapy arm:
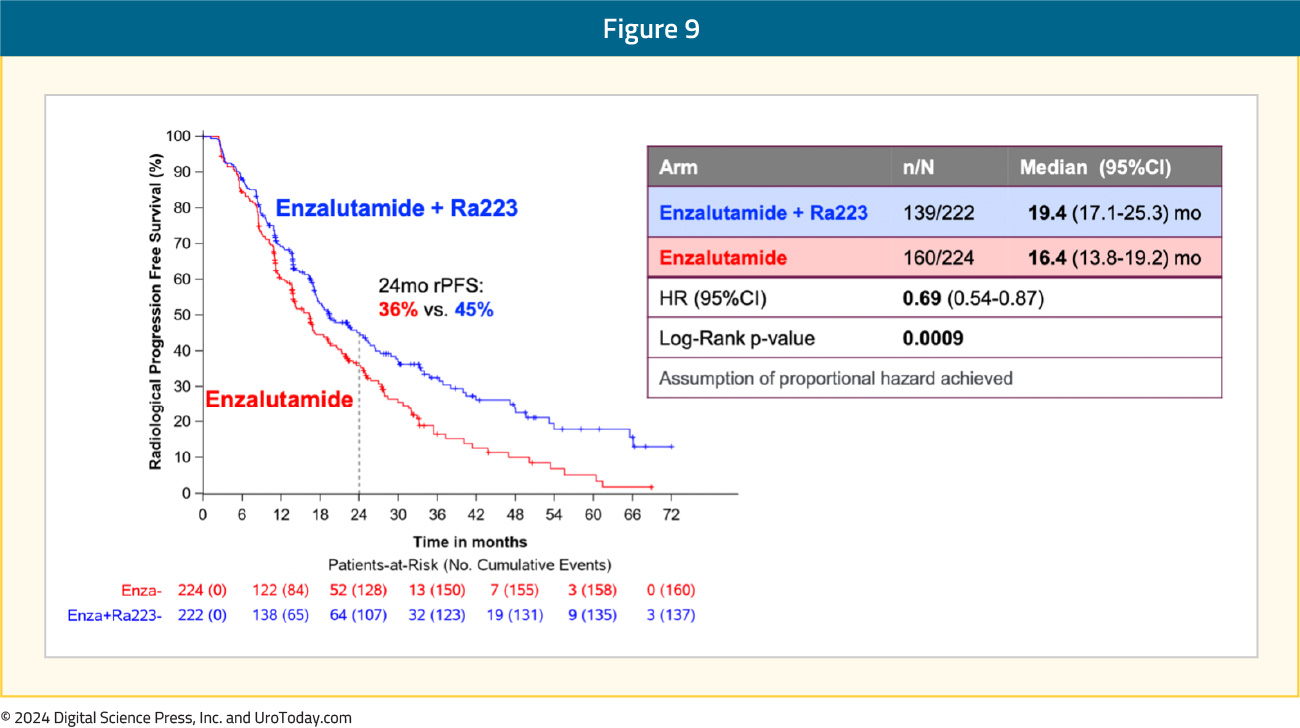
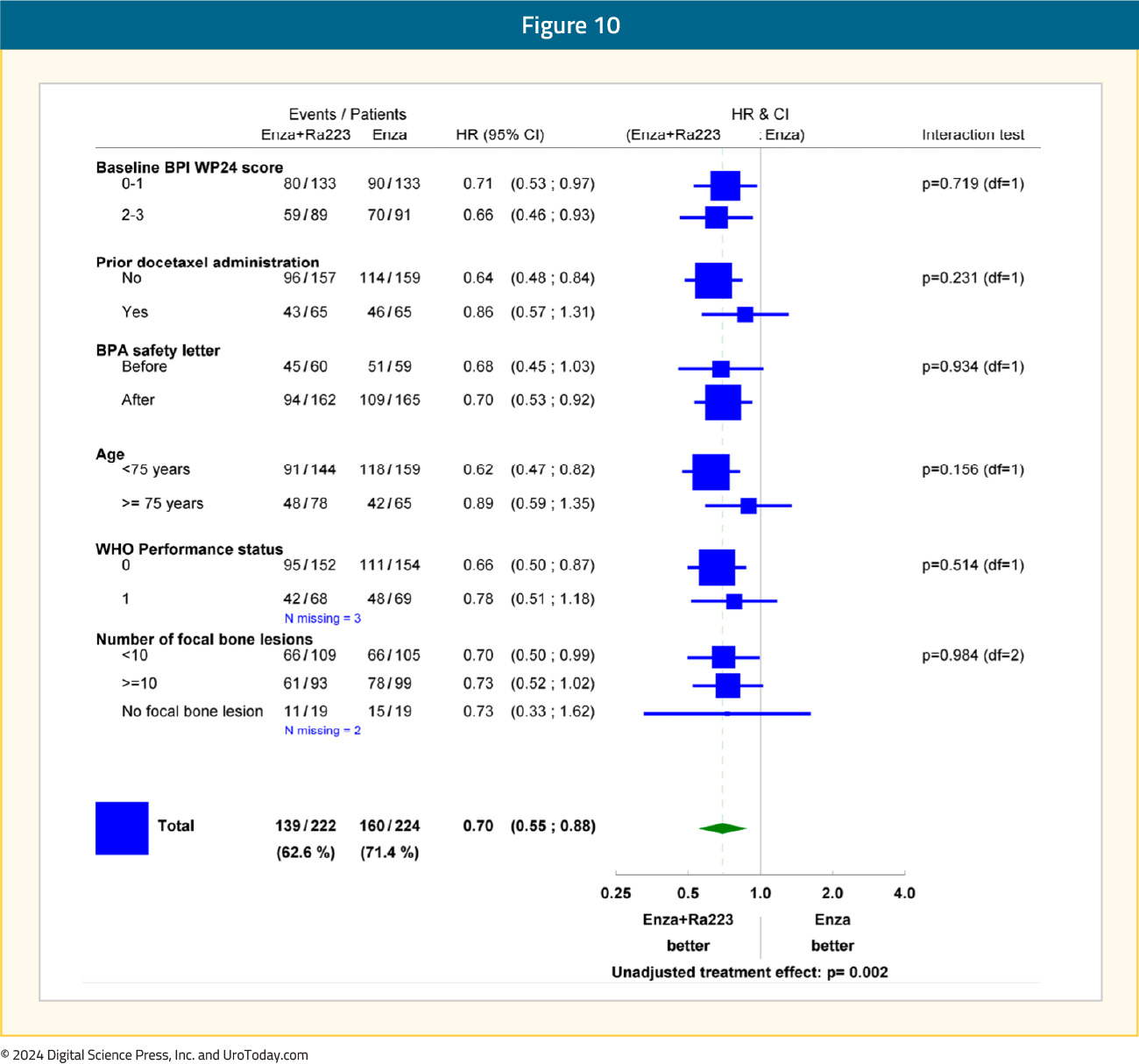
Subgroup analyses demonstrated consistent radiographic progression-free survival benefit in favor of radium-223 + enzalutamide:
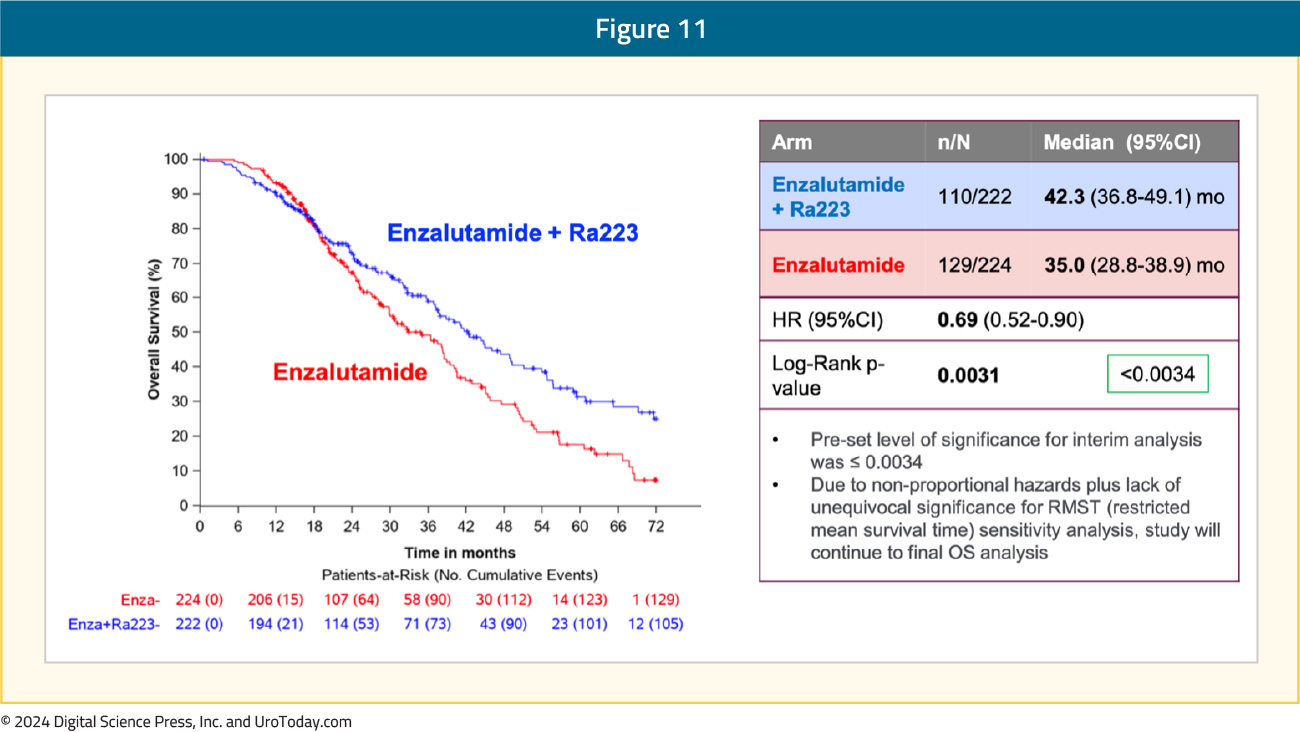
An overall survival benefit was also observed with radium-223 + enzalutamide: median overall survival of 42.3 months versus 35 months for enzalutamide monotherapy (HR 0.69, 95% CI 0.52–0.90, p=0.0031). While the p-value was below the pre-specified α threshold of 0.0034, there was evidence of non-proportional hazards. Given this, plus the lack of unequivocal significance for restricted mean survival time sensitivity analysis, the study will continue to the final overall survival analysis:
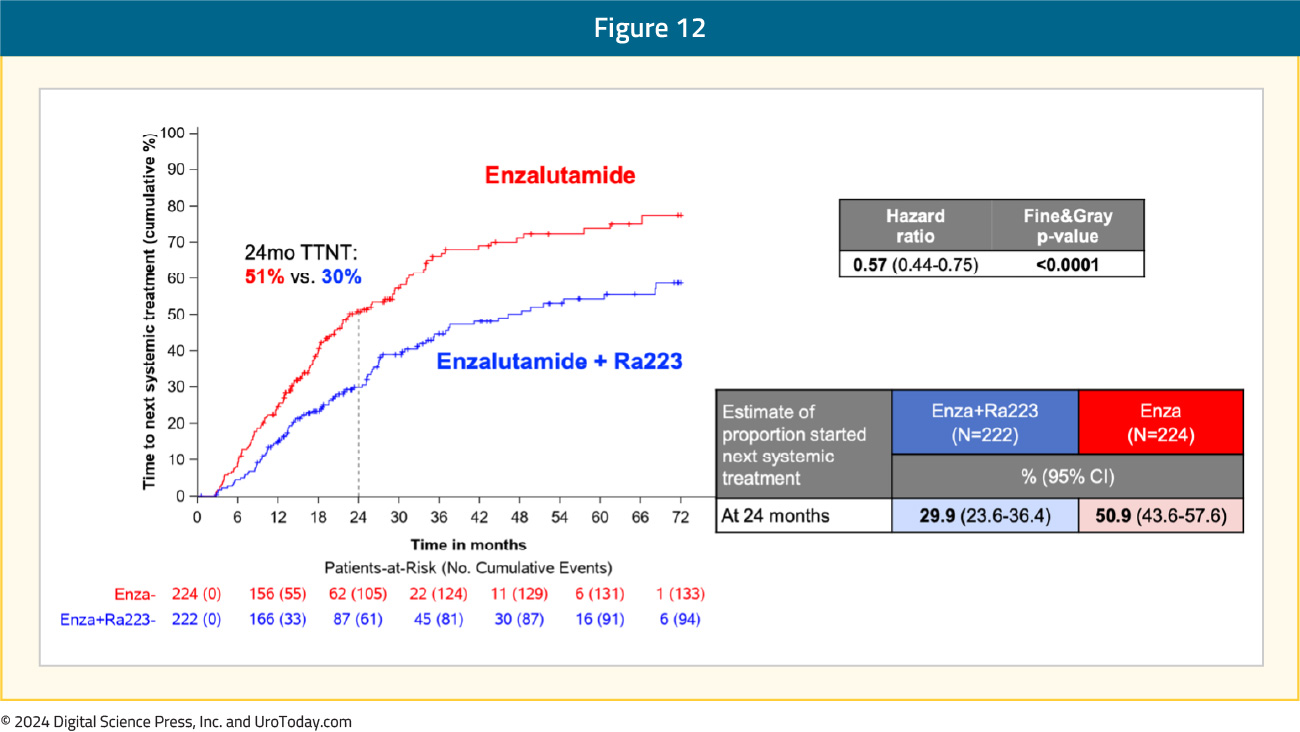
Time to next systemic treatment was also improved with the radium-223 + enzalutamide combination, with only 30% of patients in this treatment arm requiring a next-line systemic treatment at 24 months follow-up, compared to 51% of patients in the enzalutamide monotherapy arm (HR: 0.57, 95% CI: 0.44–0.75, p<0.0001):
There were no differences in time to symptomatic skeletal event, with 18% of patients in both arms having an event by 24 months follow-up. Prior to implementing the study amendment, only ~50% of patients were using bone-protecting agents. At the end of enrolment, 82–84% of patients were using bone-protecting agents:
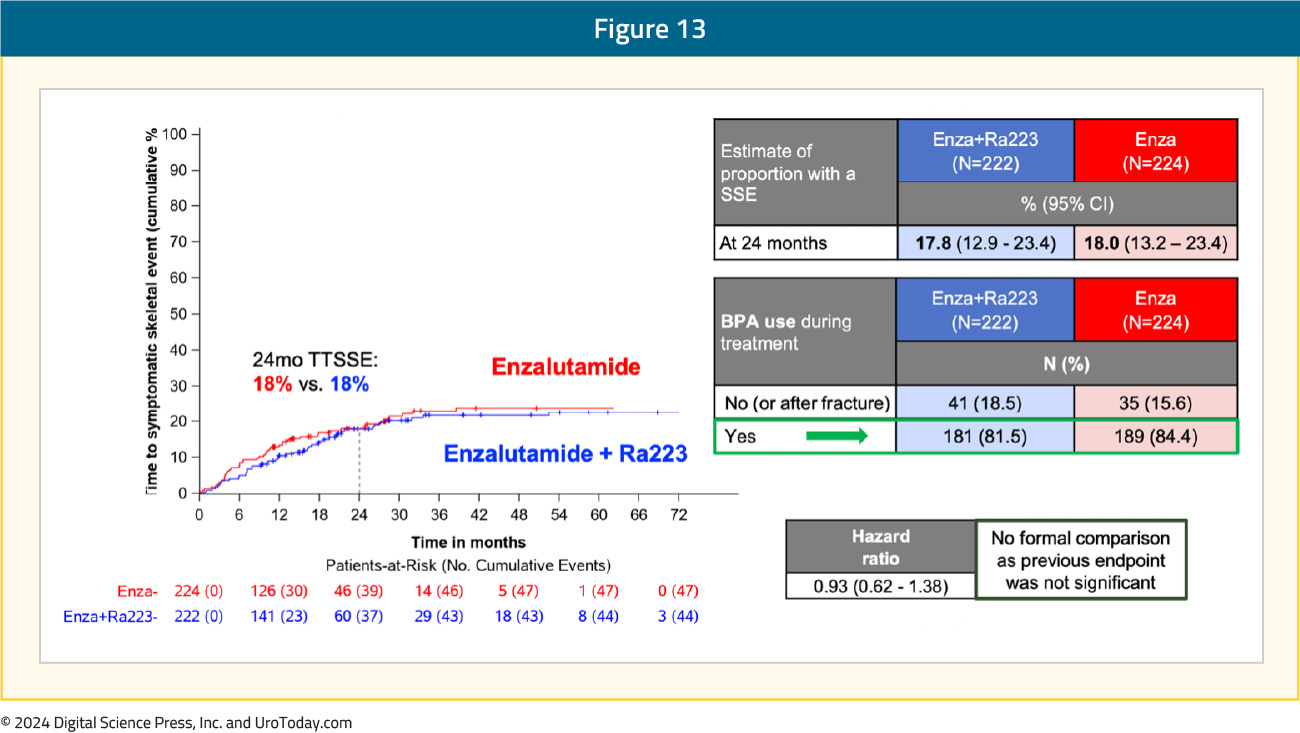
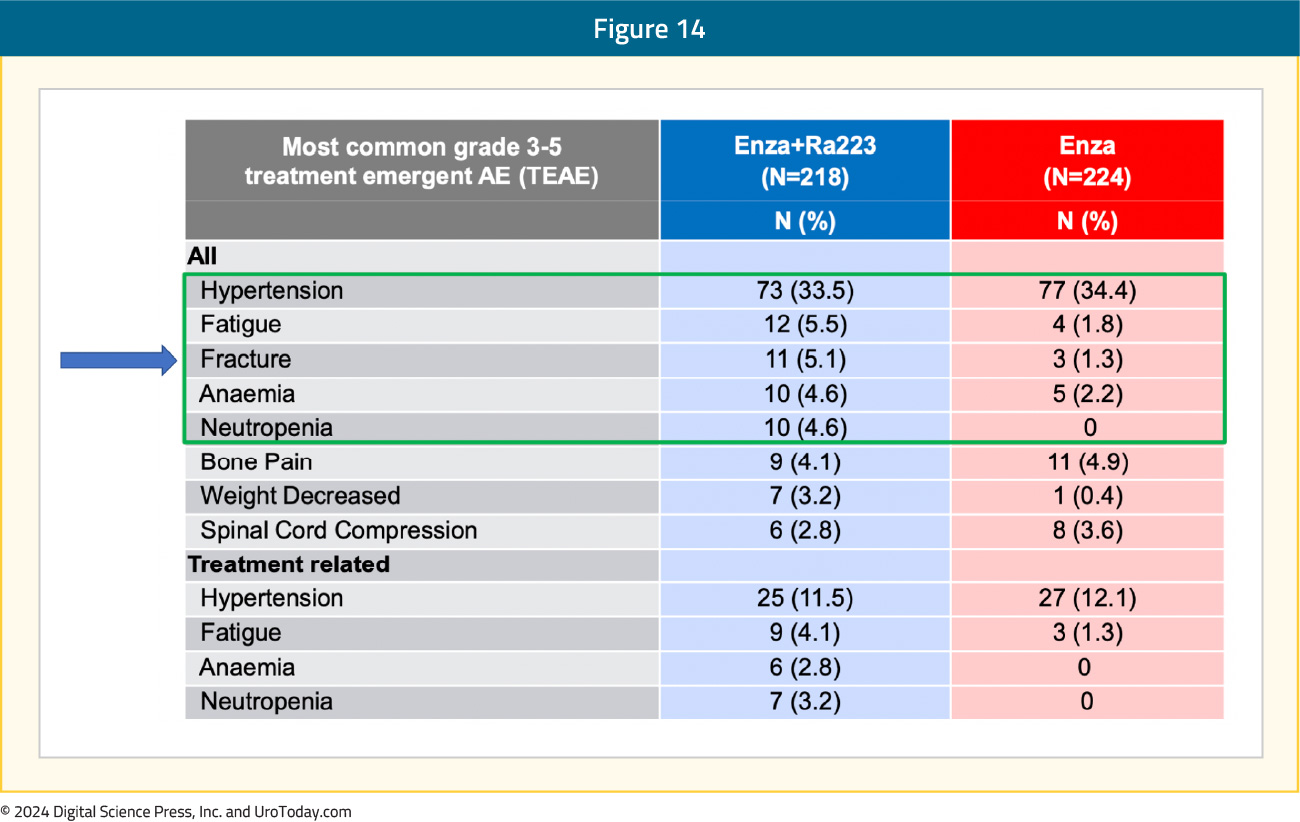
From a safety standpoint, grade ≥3 drug-related adverse events were observed in 28% and 19% of patients in the experimental and control arms, respectively. Deaths due to adverse events were observed in 3% and 2% of patients, respectively, none specifically related to the drugs used. The most common grade ≥3 treatment-emergent adverse events in the radium-223 + enzalutamide arm was hypertension (34%), fatigue (5.5%), and fractures (5.1%):
Implications of PEACE-3 and Patient Selection
The following discussion points highlight potential implications of the PEACE-3 data and may assist in patient selection.Asymptomatic/Mildly Symptomatic Bone Only Metastases: Similar to ALSYMPCA, based on the mechanism of action, radium-223 remains a treatment for bone-only metastatic mCRPC patients. However, patients in ALSYMPCA had to have symptomatic bone-metastases, whereas the criteria for PEACE-3 was only asymptomatic or mildly symptomatic bone metastases. Thus, although patients with visceral metastases would be excluded from radium-223 + enzalutamide combination therapy, the less stringent bone pain criteria in PEACE-3 may increase candidacy for combination therapy.
ADT Monotherapy in Prior Disease Spaces (nmHSPC, mHSPC): One of the exclusion criteria for PEACE-3 was a prior androgen receptor pathway inhibitor (ARPI). Indeed, combination ARPI + ADT is often used as treatment intensification in the mHSPC disease space (and also now recently in nmHSPC after presentation/approval of EMBARK).8 However, despite the approval and availability of multiple mHSPC treatment intensification strategies, there remains a clear underutilization of these combination strategies in real-world practice. There have been a multitude of studies across many countries and jurisdictions highlighting the lack of treatment intensification in mHSPC, the reasons undoubtedly multifactorial. One study from 2022 assessed data from the Adelphi Prostate Cancer Disease Specific Programme, conducted in the United States, France, Germany, Italy, Spain, the United Kingdom, and Japan between January and August 2020.8 Overall, 336 physicians participated in the survey: 58 from the US, 226 from Europe, and 52 from Japan. Globally, among 1,195 patients, ADT alone was still the most common mHSPC regimen initiated first (47.2%), followed by a novel hormonal agent at 30.7% (abiraterone: 20.7%, enzalutamide 8.2%, apalutamide 1.7%) and chemotherapy at 19.3% (mainly docetaxel at 18.5%). The highest rates of ADT only utilization was noted in Japan (78.4%) and Italy (66%), and conversely the lowest was in Spain (34%) and the United States (36%). In the United States, the most common mHSPC regimen initiated was a novel hormonal agent (41.2%), followed by ADT alone (36.3%), and chemotherapy (11.5%):
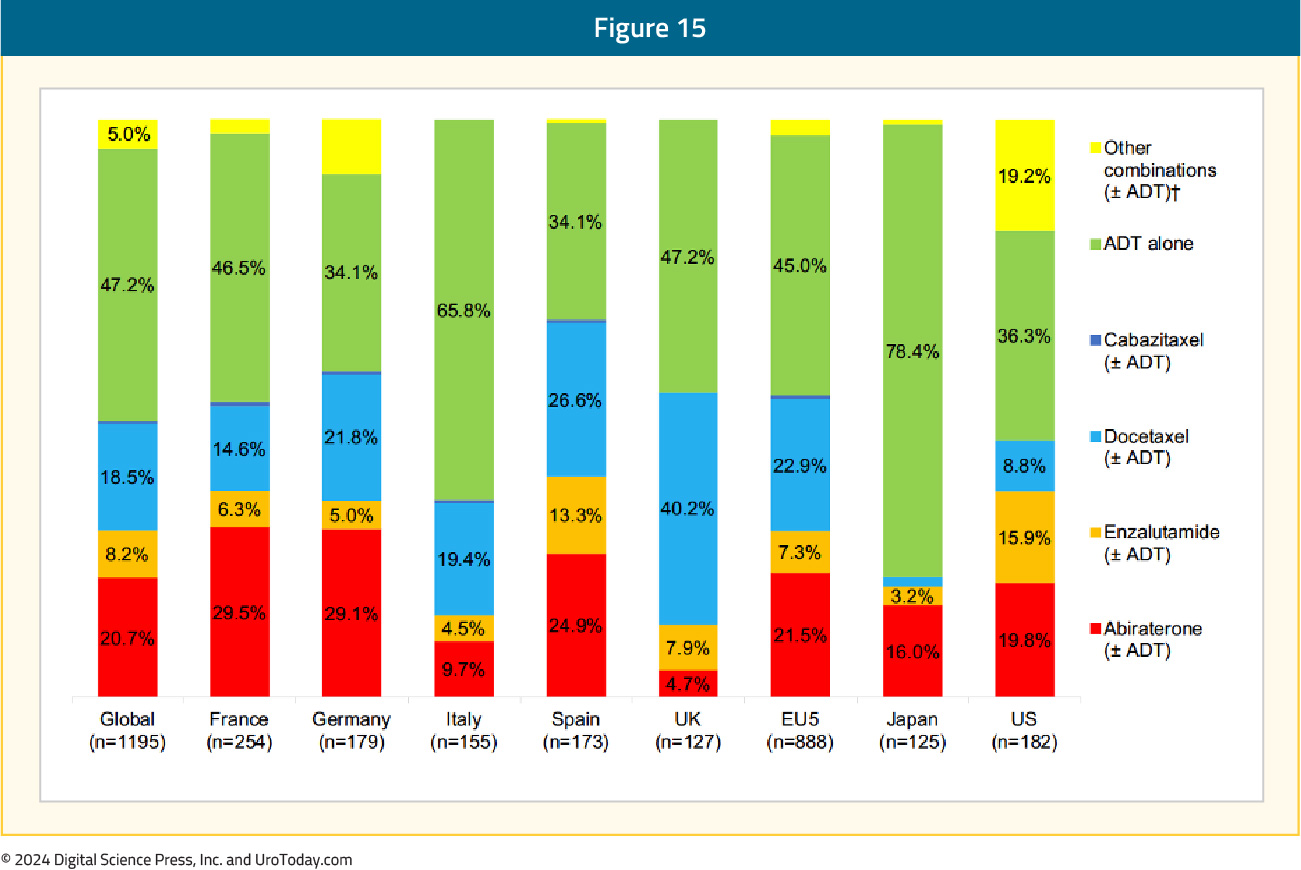
Thus, although a prior ARPI excludes patients from radium-223 + enzalutamide in first-line mCRPC, there are still ~50% of patients not receiving treatment intensification in the mHSPC setting, who would be candidates for this first-line mCRPC combination regimen.
Abiraterone in mHSPC: Abiraterone + ADT is another option in mHSPC and is still a commonly used treatment intensification modality given generic options assisting with out-of-pocket patient costs. In PEACE-3, only 2–3% of patients received abiraterone in the mHSPC setting, thus the data is limited as to whether abiraterone + ADT in mHSPC can be followed with radium-223 + enzalutamide in mCRPC.
Utilization of Bone Protective Agents: As mentioned, given the increased rate of skeletal fractures reported in the ERA-223 trial, a study amendment for PEACE-3 was introduced following the enrollment of the initial 119 patients to mandate the use of bone protective agents.7 In ERA 223, 806 patients with chemotherapy-naïve mCRPC with bone metastasis were randomized to radium-223 or placebo, in addition to abiraterone acetate. The trial was unblinded prematurely as more fractures and deaths were identified in the radium-223 arm than among patients receiving placebo. The median skeletal event-free survival was 22.3 months (IQR 17.0 to 25.8 months) among patients receiving radium-223 and abiraterone acetate and 26 months (IQR 21.8 months to 28.3 months) in patients receiving placebo and abiraterone acetate (HR 1.12, 95% CI 0.92–1.37). Fractures were more common among patients receiving radium-223 and abiraterone acetate (29%) than those receiving placebo and abiraterone acetate (11%). Subsequently, the FDA and EMA advised against this combination, which also led to bone protective agents being mandated in PEACE-3 by the IMDC. After this mandate, bone protective agent use in PEACE-3 increased dramatically from 42.6% to 82-84%. As noted in the safety data from PEACE-3 presented at ESMO 2024, fractures were still numerically higher in the radium-223 + enzalutamide arm (5.1% vs 1.3%), but much lower than observed in ERA 223. As such, for all mCRPC patients receiving radium-223 + enzalutamide, a bone protective agent must be used. However, the duration of use (2-3 years? Life-long?) and the incidence of jaw osteonecrosis from denosumab are unanswered questions from PEACE-3.
Additional Lines of Treatment if Radium-223 + Enzalutamide Fails: Certainly, the goal for patients receiving first-line radium-223 + enzalutamide for mCRPC is a durable oncologic response with appropriate quality of life. However, if a patient fails radium-223 + enzalutamide, there are many second- and third-line options. For instance, chemotherapy (docetaxel or cabazitaxel), PARP inhibitors, and radioligand therapy would be several potential treatment options. As mentioned, RALU has provided data to suggest that radium-223 followed by 177Lu-PSMA-617 is safe and feasible.4
Conclusions:
The results from PEACE-3 support the combination of radium-223 + enzalutamide in combination with a bone protective agent as a new first-line treatment option for mCRPC in patients who have not received a prior ARPI. Following regulatory approval, our job as clinicians will be to select appropriate patients for this first-line mCRPC combination therapy. Additionally, ongoing phase III trials are assessing radium-223 in mCRPC, including DORA, which is evaluating the combination of docetaxel + radium-223 and RADIANT, which is evaluating radium-223 in the post-ARPI and docetaxel setting: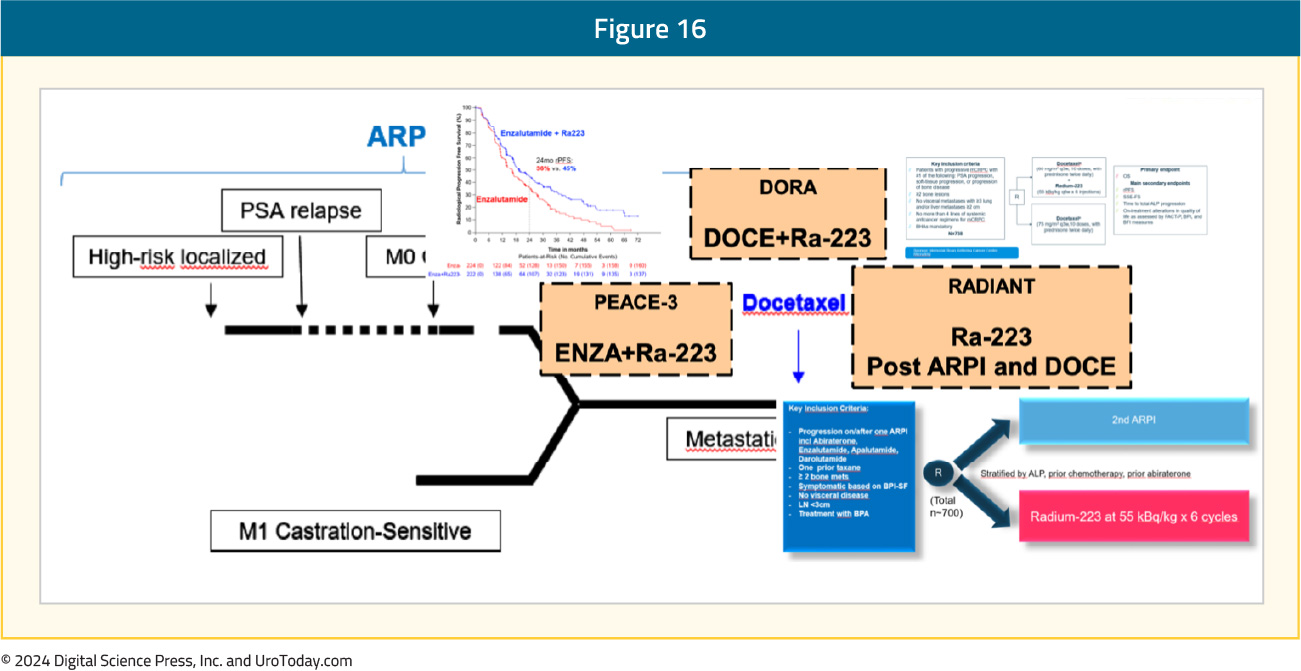
Written by: Rashid Sayyid, MD, MSc, Robotic Urological Oncology Fellow, Department of Surgery, Section of Urology, University of Southern California, Los Angeles, CA and Zachary Klaassen, MD, MSc, Assistant Professor Surgery/Urology at the Medical College of Georgia at Augusta University, Wellstar MCG Health, Augusta, GA
Published October 2024


