Androgen receptor inhibition remains the mainstay treatment of advanced prostate cancer. However, therapeutic resistance to androgen receptor inhibition is almost universal. Prostate cancer cells can develop resistance to androgen ablation through an adaptive marked upregulation of androgen receptors over time in response to low-androgen conditions. This upregulation can make these cells vulnerable to supraphysiologic testosterone exposure. Bipolar androgen therapy (BAT) has been proposed as an approach to overcome androgen receptor therapeutic resistance. Rapid cycling between polar extremes of supraphysiologic and near-castrate serum testosterone in asymptomatic men with metastatic castrate-resistant prostate cancer (mCRPC) has been proven safe and effective. There are five unique features to BAT:
- Has an acceptable safety profile
- Can induce objective responses
- Can sensitize cancer cells to androgen receptor inhibition
- Can improve patient quality of life
- Is inexpensive
In this Center of Excellence article, we will discuss the underlying rationale for BAT, how BAT can be performed in clinical practice, and potential candidates for BAT within the context of the current evidence from the literature.
Rationale for BAT
Prostate cancer cells adapt to chronic low testosterone conditions by upregulating androgen receptor activity through overexpression, gene amplification, and expression of transcriptionally active receptor variants that lack the ligand-binding domain.1-4 Androgen receptor levels may increase by 30 to 90-fold in CRPC cell lines and clinical samples, compared to normal prostate cells.5 While this marked upregulation of the androgen receptor can cause castration resistance, it also creates a therapeutic vulnerability to treatment with supraphysiologic androgen, which can lead to growth arrest or cell death in CRPC cell models.6-8 However, over time, prostate cancer cells become resistant to supraphysiologic androgens through downregulation of the androgen receptor, making them once again sensitive to androgen deprivation therapy (ADT).9
How is BAT Performed in Clinical Practice?
The term ‘bipolar’ refers to the rapid cycling between two polar extremes: from supraphysiologic back to near-castrate serum testosterone levels. It can be achieved by administering testosterone cypionate at the US Food and Drug Administration (FDA)–approved dose of 400 mg intramuscularly once every 28 days, while maintaining continuous testosterone suppression via surgical castration or luteinizing hormone–releasing hormone (LHRH) agonists or antagonists. Testosterone can also be delivered via transdermal, transbuccal, intranasal, or oral preparations. However, achieving supraphysiologic levels of testosterone with these preparations is likely to be cost-prohibitive and require non-FDA-approved dosing.10
Clinical Trials Assessing BAT
TRANSFORMERPublished in The Journal of Clinical Oncology in 2021, TRANSFORMER is a multicenter, open-label, phase II trial that randomized 195 men with mCRPC who had disease progression following treatment with abiraterone to either BAT (n = 94) or enzalutamide 160 mg once daily (n = 101):
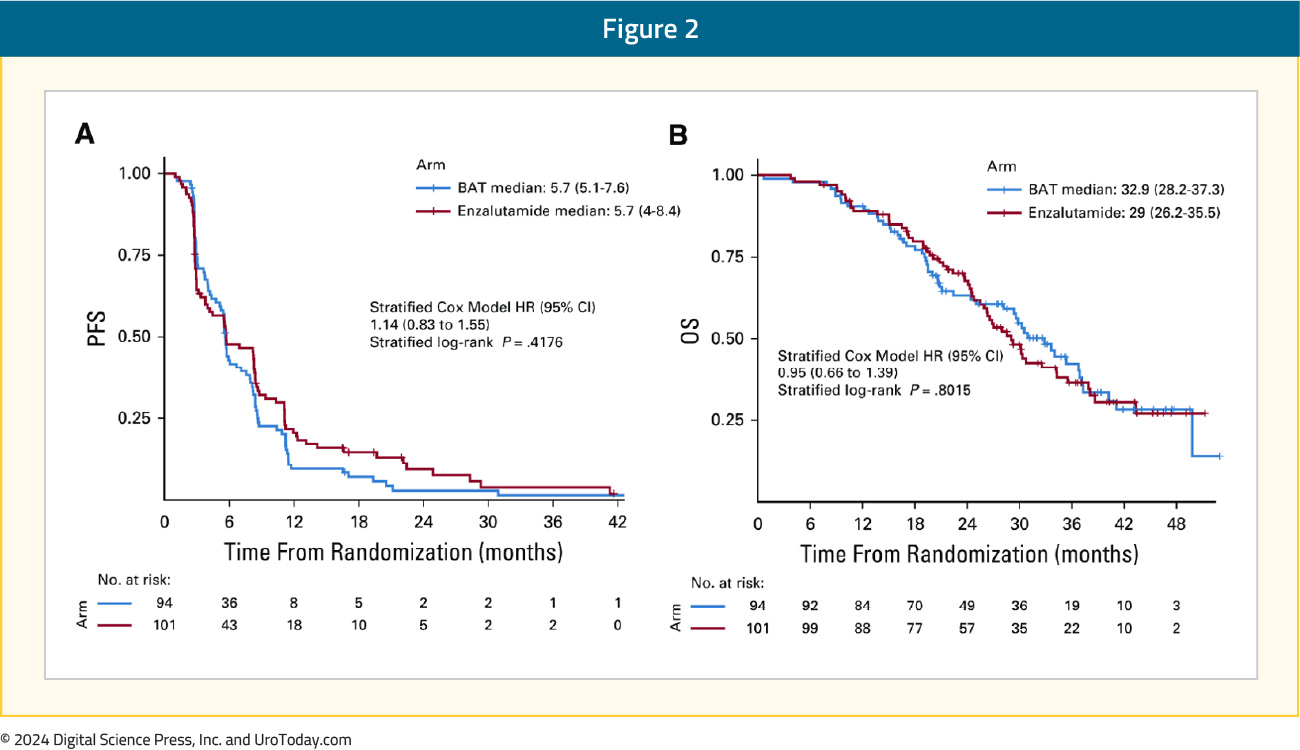
At a median follow-up time of 32 months, there was no difference in the primary study outcome, progression-free survival (BAT: 5.6 months versus enzalutamide: 5.6 months; HR: 1.13, p = 0.45). Additionally, there was no difference in overall survival (BAT: 33 months versus enzalutamide: 29 months; HR: 0.95, p = 0.80):
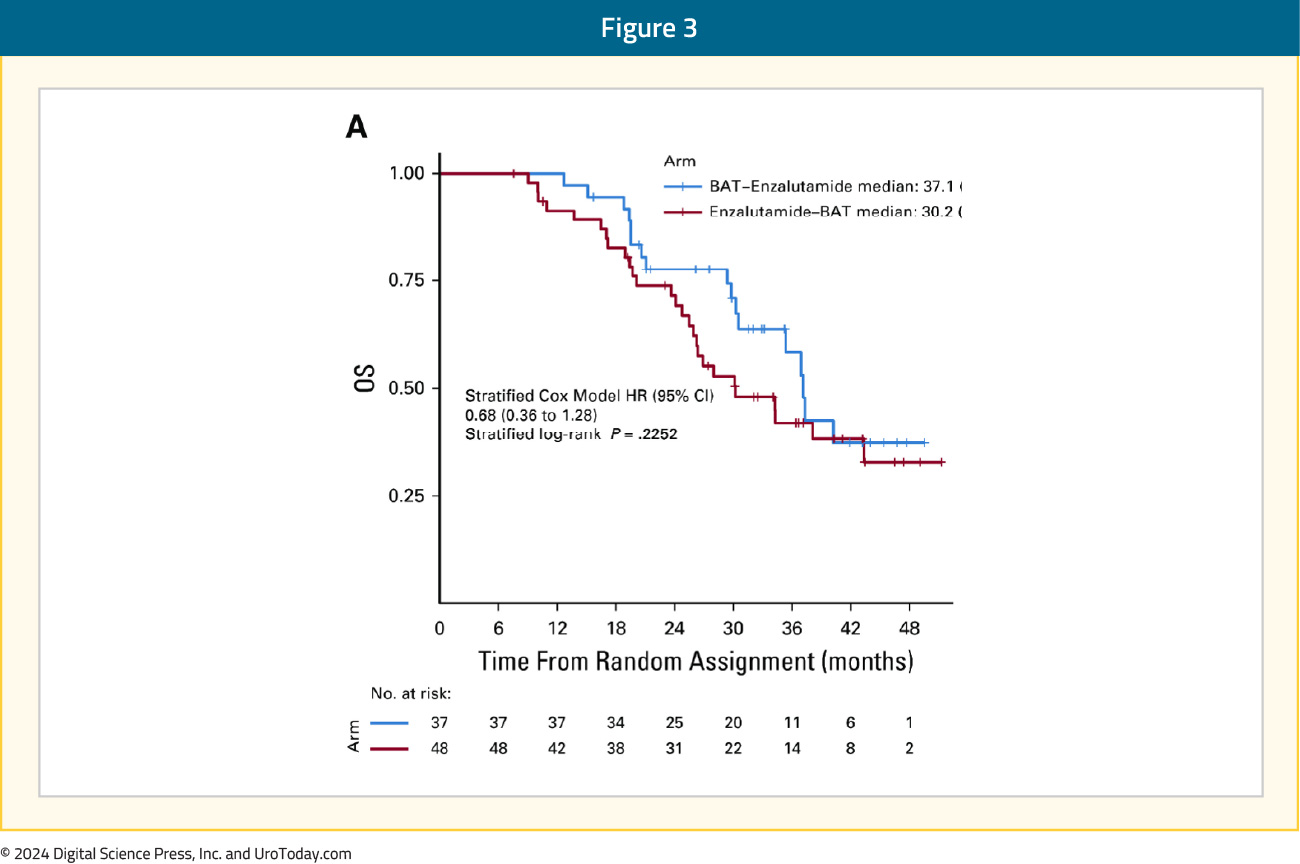
Patients who remained asymptomatic and continued to meet eligibility requirements were provided the opportunity to cross over, after a 28-day washout period, to the alternate treatment at time of progression. Most patients crossed over due to radiographic progression (90–95%). Crossover to enzalutamide following BAT was associated with greater benefits than crossover to BAT following enzalutamide. PSA50 responses occurred in 77.8% of patients crossing over to enzalutamide, compared to 23.4% in those crossing over to BAT. The progression-free survival from randomization through crossover (PFS2) was 28.2 months for BAT → enzalutamide versus 19.6 months for enzalutamide → BAT (HR: 0.44; p = 0.02). The overall survival was 37.1 months for enzalutamide → BAT versus 30.2 months for enzalutamide → BAT (HR: 0.68, p = 0.23):
The incidence of serious adverse events was similar in both arms (~20%). Patients in the enzalutamide arm experienced fatigue more frequently (grade ≥3: 7.2% versus 0% for BAT) and back pain (grade ≥3: 7.2% versus 3.4% for BAT). Enzalutamide was associated with a higher frequency of constitutional symptoms such as anorexia, depression, anxiety, insomnia, headache, and generalized muscle weakness, as well as gastrointestinal complaints. BAT was associated with increased sexual side effects (hot flashes, breast tenderness, and gynecomastia) and musculoskeletal complaints (peripheral edema and generalized musculoskeletal pain).11
The presence of androgen receptor (AR) alterations in circulating tumor DNA (ctDNA) may help identify patients who might benefit from BAT. Presented at ASCO 2024, ctDNA from sequenced patients (tested somatic cohort) in the TRANSFORMER trial was analyzed. This cohort included 62 patients, selected based on circulating tumor cells (CTC) and blinded to trial data. Of these, 33 out of 62 patients (53%) had an AR alteration, which was only linked to trial data after ctDNA mutation calling. The respective mutations were:
- ctDNA WES, AR mutation (n=7, t878a or l702h)
- ctDNA WGS, AR amplification (n=27)
Patients with no AR alterations treated with BAT exhibit significantly shorter progression free survival (p = 0.006) and overall survival (p = 0.025) compared to those treated with enzalutamide:
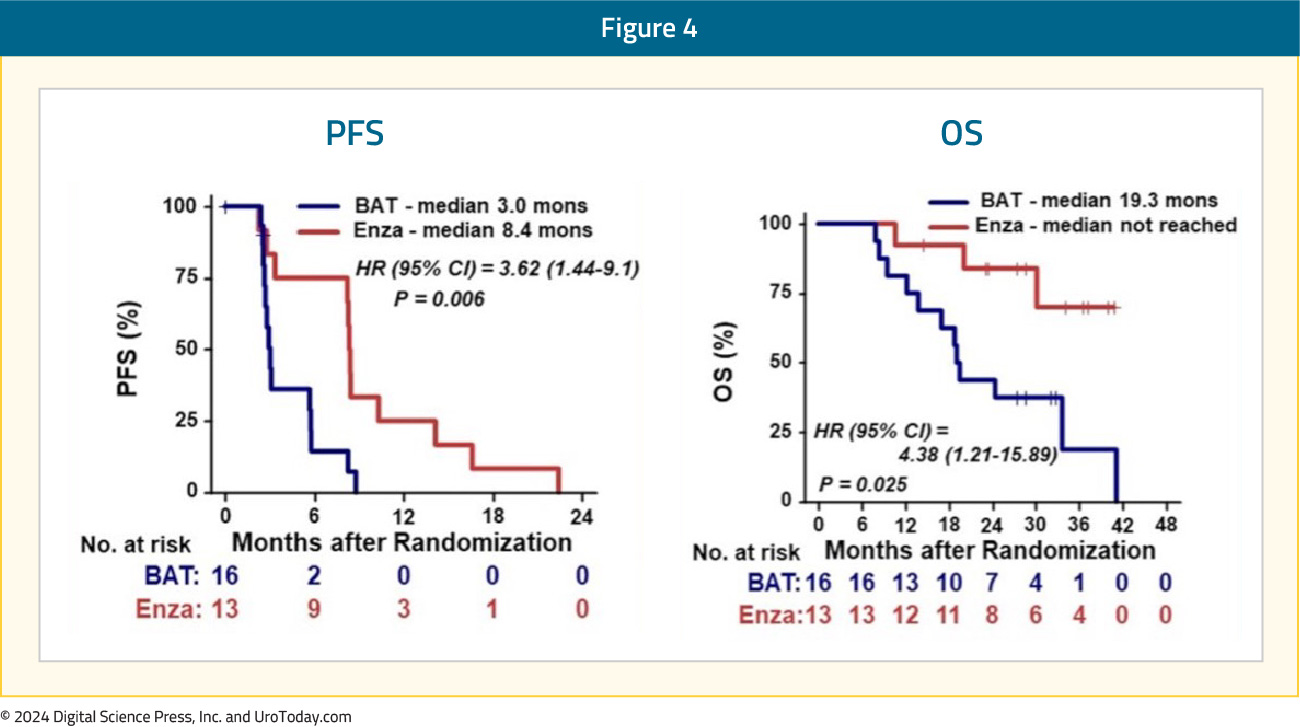
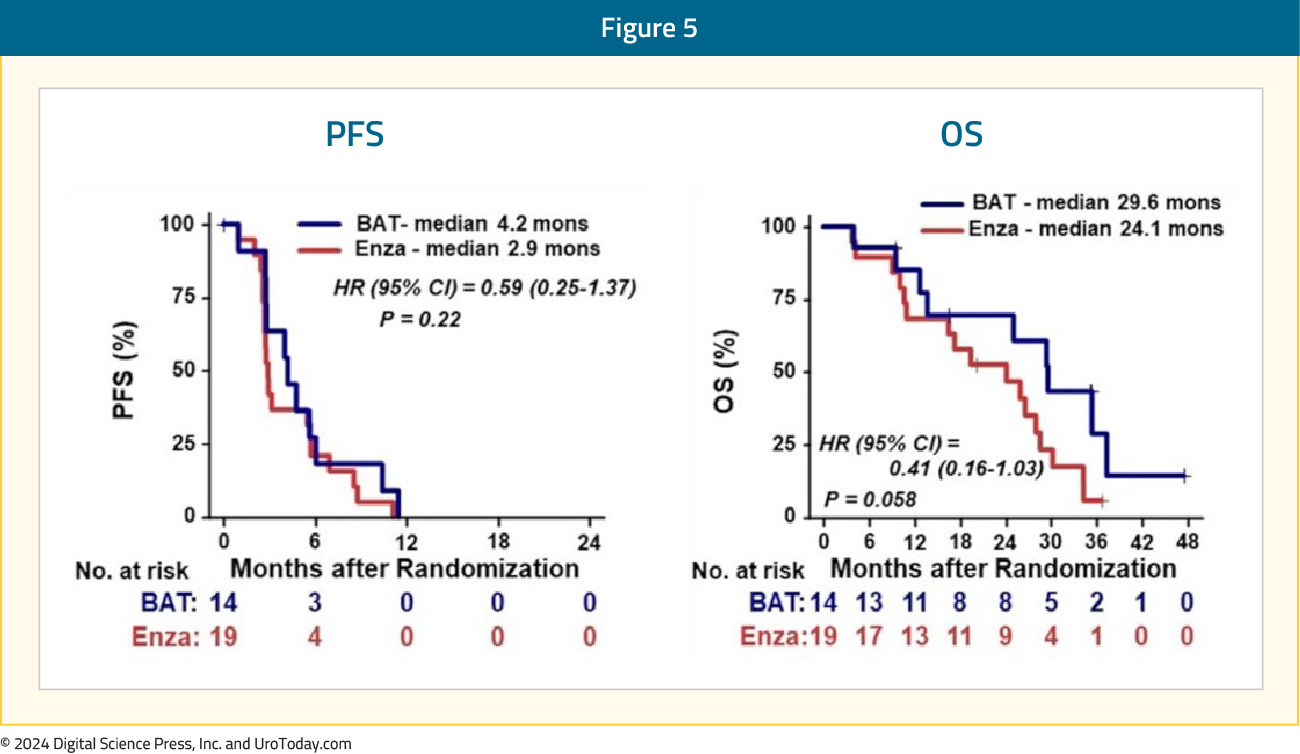
However, patients with AR alterations treated with BAT trended toward prolonged progression free survival (p = 0.22) and overall survival (p = 0.058) versus enzalutamide, although not significant:
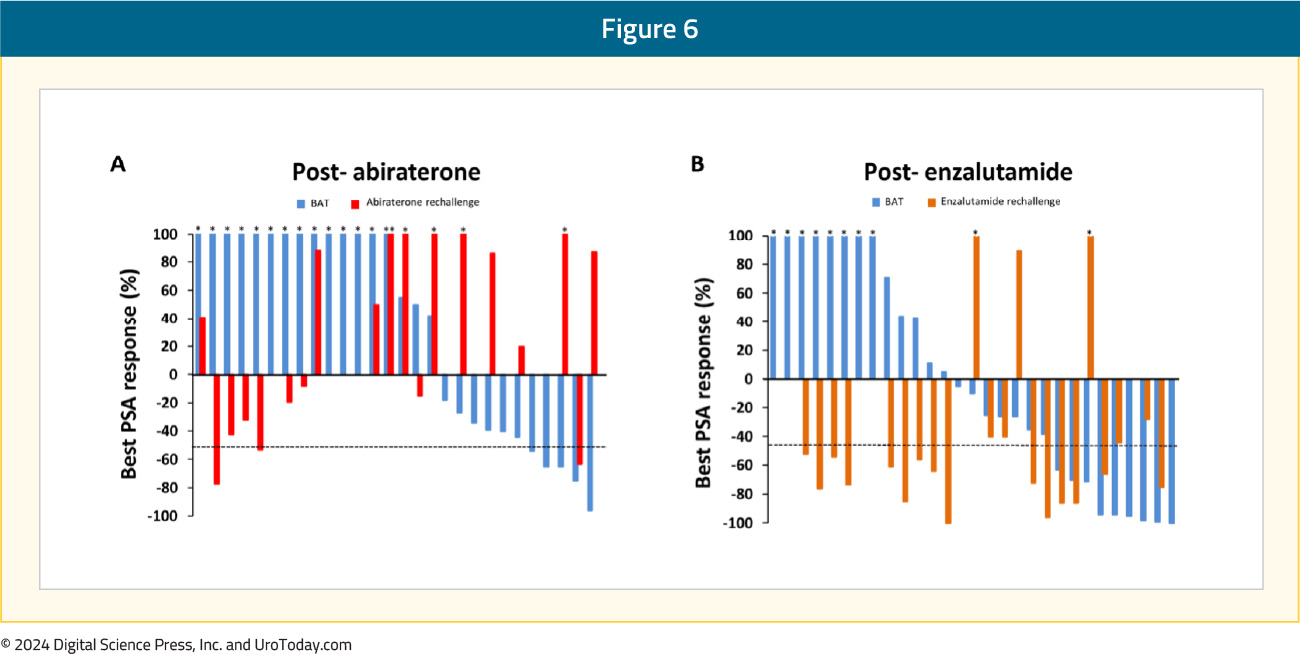
RESTORE is a single arm phase II trial of BAT in 59 mCRPC patients that experienced disease progression following abiraterone (n = 29) or enzalutamide (n = 30) treatment. Following progression on BAT, patients were re-challenged with their most recent androgen receptor pathway inhibitor (ARPI). No prior chemotherapy for mCRPC was permitted.
PSA50 responses were observed in 30% and 17% of the enzalutamide and abiraterone pre-treated cohorts, respectively (p = 0.40). PSA50 responses to the ARPI rechallenge were also higher in the enzalutamide pre-treated cohort (68% versus 16%, p = 0.001). The median time from enrollment to progression following re-challenge with the ARPI was comparatively longer in the enzalutamide-treated patients (12.8 versus 8.1 months, p = 0.04).
The most common adverse events during treatment with BAT were musculoskeletal pain and peripheral edema. Two patients experienced grade 3 toxicities (musculoskeletal pain and alkaline phosphatase elevation), which resolved with treatment delay. One patient developed grade 4 disseminated intravascular coagulation at progression and was not re-challenged. No patients were removed from the study for drug toxicity to BAT, and there were no treatment-related deaths.12
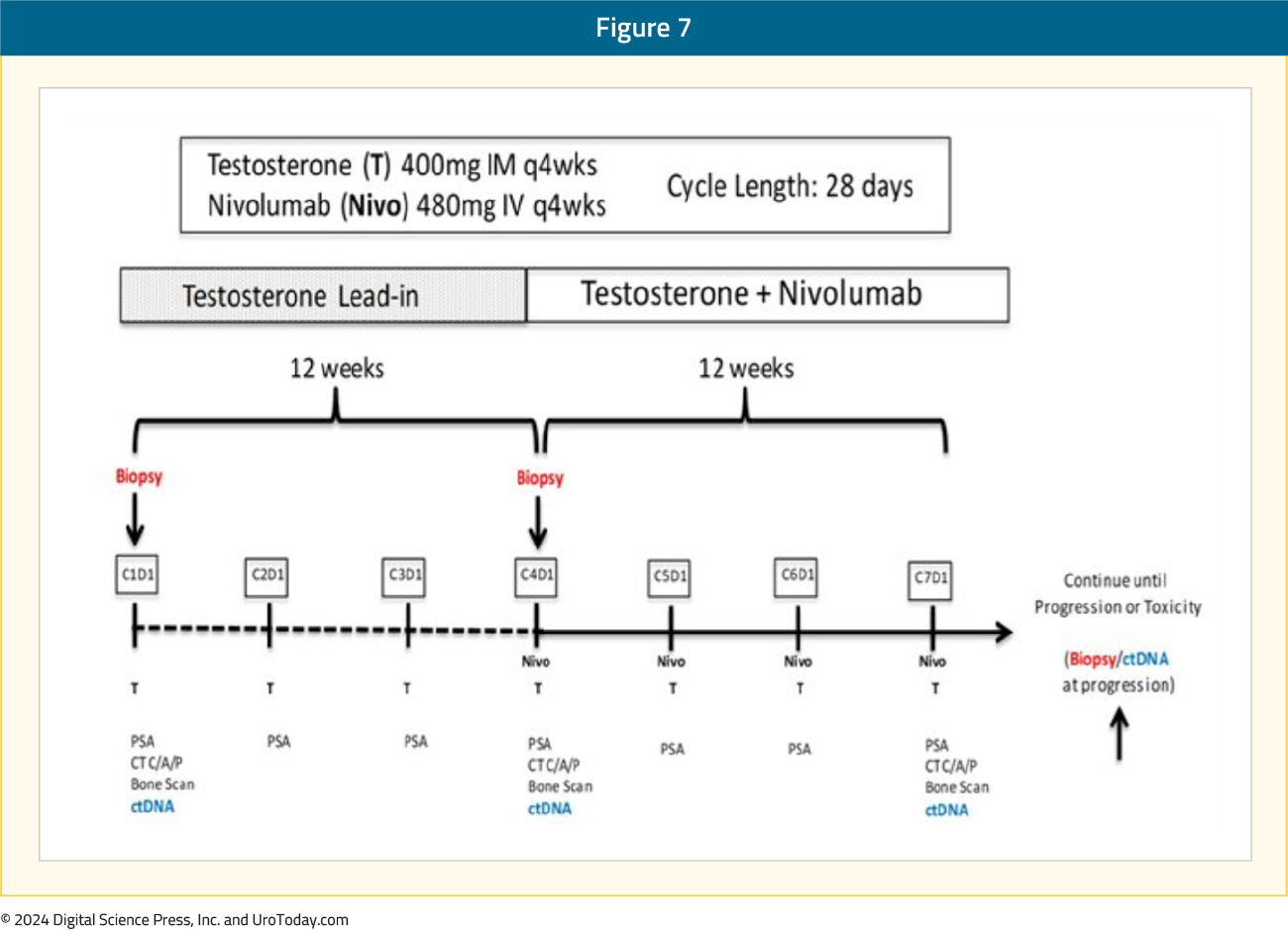
COMBAT: BAT + NivolumabPre-clinical studies have demonstrated that supraphysiologic testosterone exposure could activate interferon response pathways.13 This, coupled with limited clinical data demonstrating excellent outcomes for patients treated with BAT followed by BAT + nivolumab,14 served as the rationale for the COMBAT study. This is a single arm phase II trial of 45 heavily pre-treated mCRPC patients who received BAT monotherapy for three cycles, followed by combination BAT + nivolumab:
Overall, 53% of patients had received ≥2 prior ARPIs, and 44% had received prior taxane chemotherapy. All patients received at least one dose of BAT. Eight patients received only BAT, and experienced disease progression prior to the start of nivolumab. The primary endpoint of a confirmed PSA50 response rate was met and estimated at 40% (null hypothesis: 25%; 95% CI: 25.7–55.7%, p = 0.02). There were 16/18 PSA50 responses achieved prior to the addition of nivolumab, and the objective response rate was 24%:
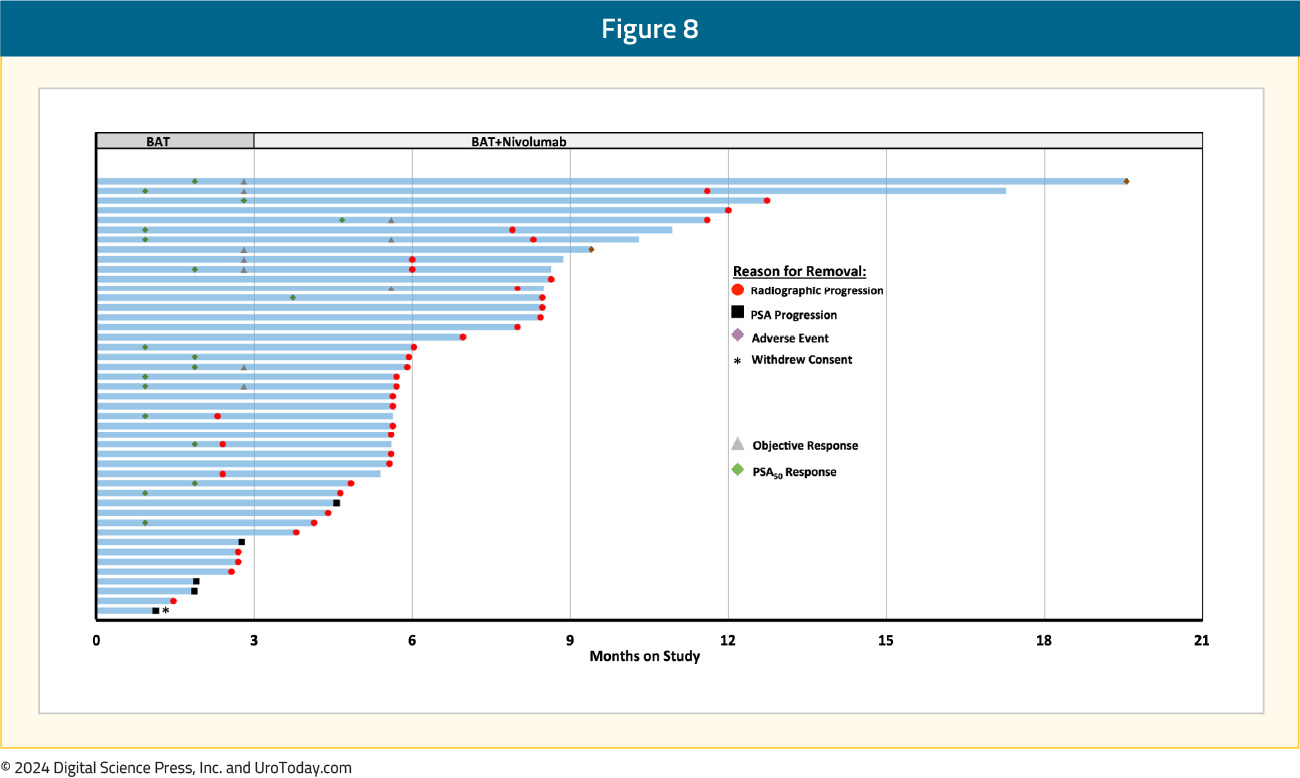
At a median follow-up of 17.9 months, the median radiographic progression-free survival was 5.6 (95% CI: 5.4–6.8) months, and the median overall survival was 24.4 (95% CI: 17.6–31.1) months:
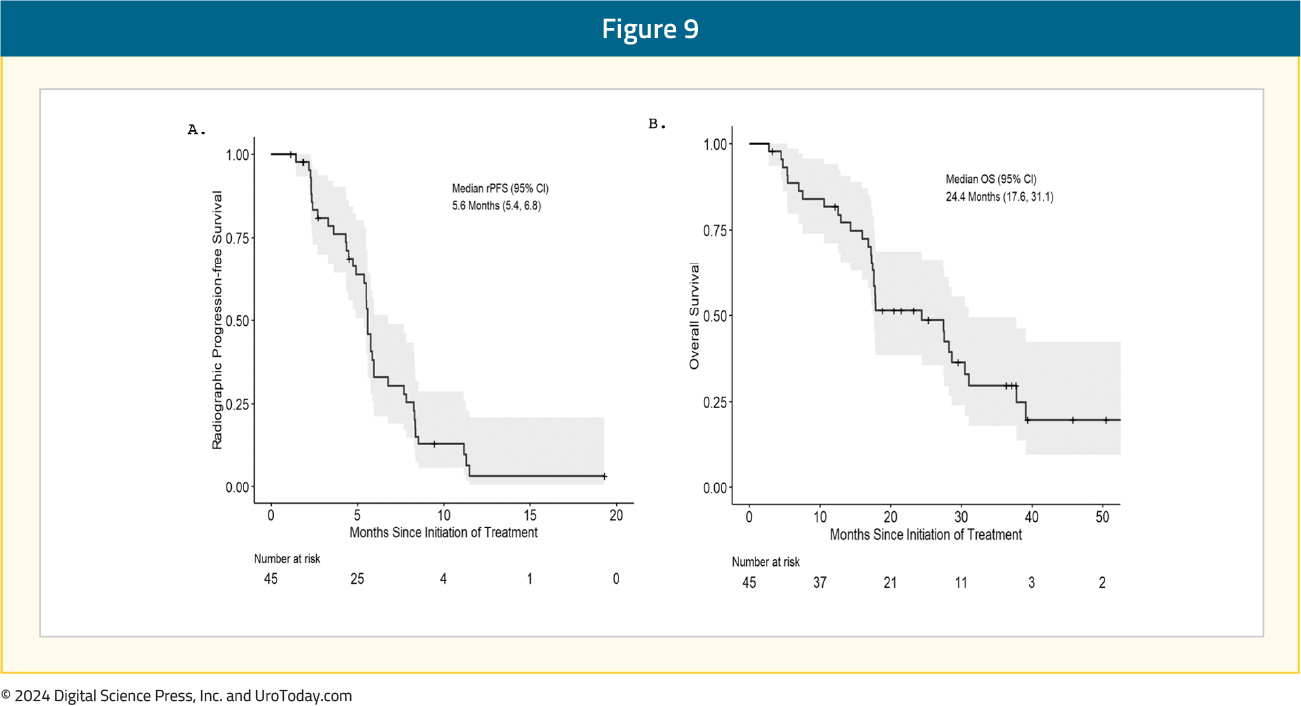
The combination of BAT + nivolumab was well-tolerated, resulting in only five (11%) drug-related, grade 3 adverse events (musculoskeletal pain, edema, lipase elevation, fatigue, pericarditis, cardiomyopathy; 1 each).15
BAT + OlaparibIt has been theorized that BAT’s effects may be mediated, at least partially, by the induction of DNA damage. This suggests a synergistic mechanism of action with PARP inhibitors, such as olaparib. A single center phase II trial evaluated the combination of BAT + olaparib (300 mg twice daily) in 36 mCRPC patients with disease progression following prior abiraterone and/or enzalutamide (10/36 received both). In this trial, a PSA50 response was observed in 47% of patients:
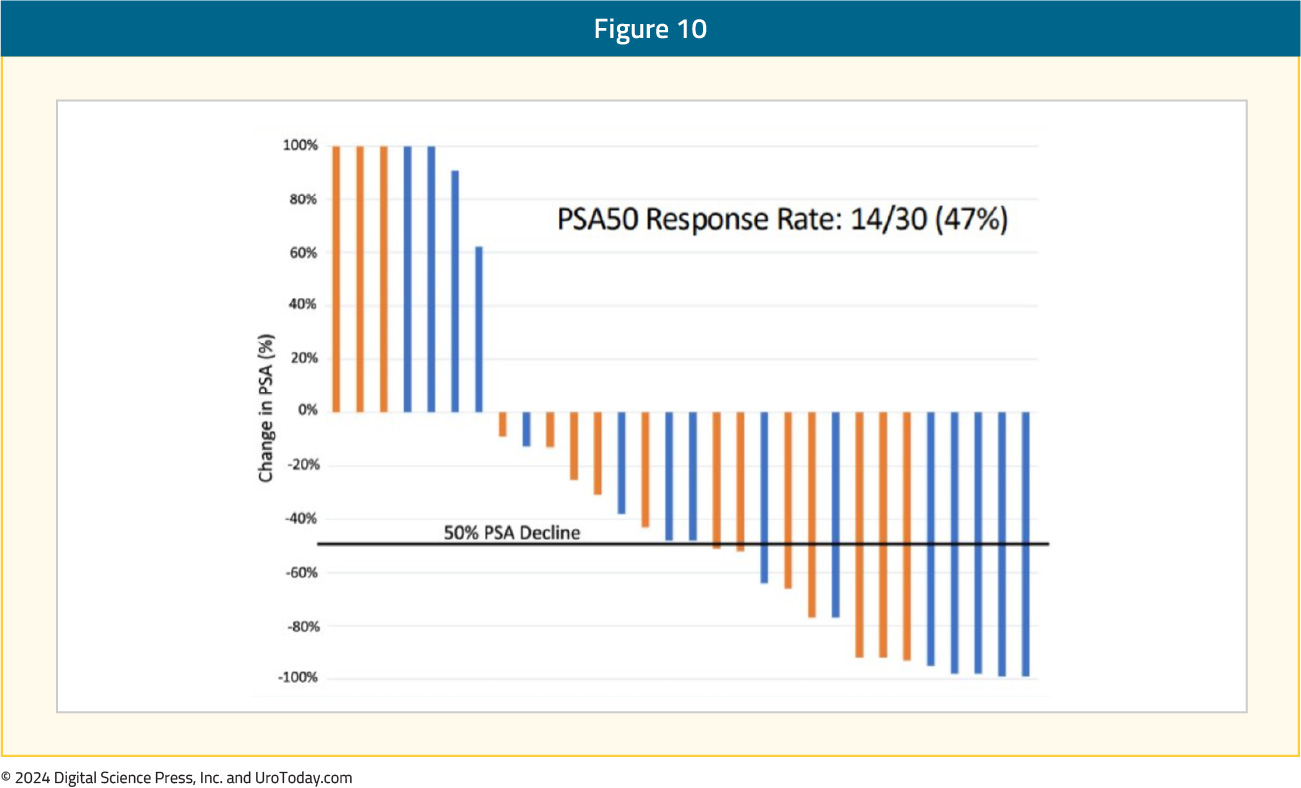
At a median follow-up of 22.7 months, the median progression-free survival was 12.6 months. In terms of toxicity, five patients had grade ≥3 treatment-related adverse events, including one stroke (Grade 4) and one myocardial infarction (Grade 5). Following a protocol amendment to exclude patients with a history of a myocardial infarction, no further cardiovascular events were observed.16
BAT in Current mCRPC Treatment Paradigm
While BAT represents an exciting, novel therapeutic approach for mCRPC patients, it does not appear that BAT is ready yet for ‘prime time’ based on the current body of literature. The only randomized trial of BAT in mCRPC patients (TRANSFORMER) has failed to demonstrate a survival benefit for an ARPI switch in patients with disease progression following prior ARPI therapy. An ARPI switch is no longer considered an acceptable standard of care approach for the treatment of such patients in contemporary practice. When we consider the plethora of approved/emerging options for mCRPC patients in the post-ARPI setting, including taxane chemotherapy (docetaxel and cabazitaxel), PARP inhibitors (olaparib and rucaparib for patients with homologous recombination repair mutations and BRCA mutations, respectively), radionuclides (177Lu-PSMA, 225Ac), it is hard to ‘justify’ the use of BAT for mCRPC patients at the current time. This is reflected in the latest update of the NCCN prostate cancer guidelines that do not include BAT as a treatment option for mCRPC patients, irrespective of prior therapy received.17 Nonetheless, it does appear that novel combination approaches of BAT with either PARP inhibitors or anti-PD-1/L-1 agents may have promising efficacy outcomes in heavily pre-treated mCRPC patients and may represent a novel therapeutic approach for such patients, pending further prospective evaluation. We look forward to eventual data from the following clinical trials with BAT that are open and actively accruing:
- STEP-UP (sequencing BAT and enzalutamide)
- APEX (sequencing BAT + Difluoromethylornithine (DFMO) with enzalutamide)
- BATRAD (BAT + Radium-223)
- AcroBAT (oral testosterone)


