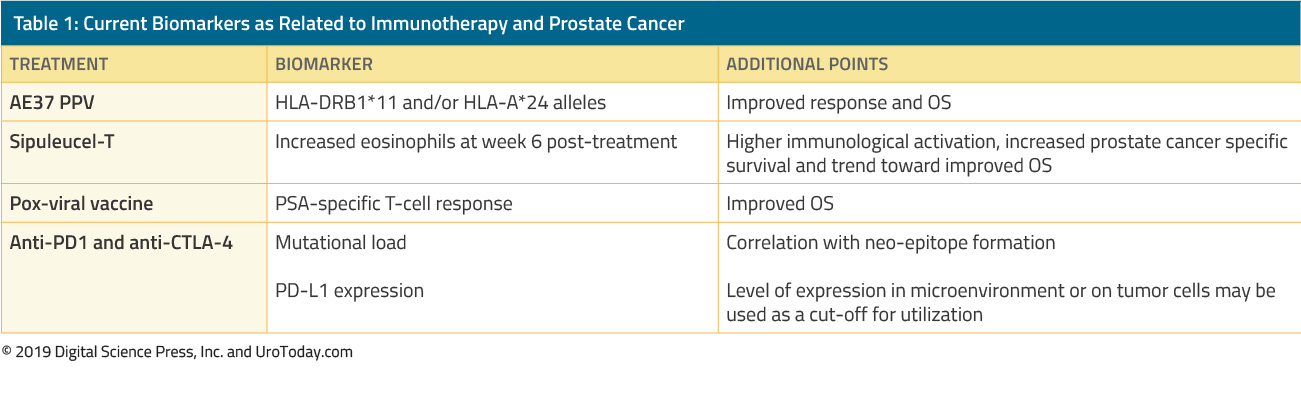
The Opioid Crisis in Urology
The Opioid Crisis in Urology
A study in 2011 from the University of Utah provided surveys to consecutive patients undergoing surgery during a 3-month time frame to assess perception of pain control, type and quantity of medication prescribed, quantity of leftover medication, refills needed, disposal instructions, and surplus medication disposition.2 Surveys were performed 2 to 4 weeks postoperatively, and with the exception of the investigators, prescribing physicians had no prior knowledge of the study. Among the 586 patients undergoing surgery, 47% participated in the study. Hydrocodone was prescribed most commonly (63%), followed by oxycodone (35%); 86% of the patients were satisfied with pain control. Of the dispensed narcotics, only 58% were consumed, while 12% of patients requested refills. A total of 67% of patients had surplus medication from the initial prescription and an alarming 92% received no disposal instructions for surplus medication. Among patients with leftover medication, 91% kept the medication at home while 6% threw it in the trash, 2% flushed it down the toilet, and less than 1% returned it to a pharmacy. Indeed, the retained surplus of medication provides a readily available source of opioid excess.In a prospective observational study of 155 opioid naïve patients who underwent a major prostate or kidney operation, investigators conducted a telephone survey 3-4 weeks postoperatively to assess the number of 5 mg oxycodone-equivalents prescribed, opioid use, and disposal.3 Most patients were male (86%), most were married (74%), the median was age 64 (IQR 59-70) years of age, and the majority were Caucasian (84%). Most patients reported social alcohol use (56%), but most denied current tobacco use (77%) or current and/or previous drug use (76%). Opioid prescribing exceeded use from 1.9- to 6.8-fold for all procedural categories. Overall, a total of 4,065 oxycodone-equivalents were prescribed during the study and 60% of pills prescribed went unused, resulting in 2,622 excess pills in the community.
Unfortunately, opioid overprescribing is not limited to the adult population, as it has also been demonstrated in pediatric urology patients. At the University of North Carolina, 117 pediatric urology patients’ parents were contacted with 39% completing a two-week post-operative telephone survey. The three most common pediatric urology procedures were inguinal hernia repair (n = 39), circumcision (n = 27), and cystoscopy (n = 16). Across all procedures, there was an average excess of 9.8 doses prescribed, corresponding to an over-prescription rate of 64%. Among the patients prescribed opioids, 41 (62%) had leftover opioid medication two weeks postoperatively. Thirty-two of 41 (78%) patients did not dispose of their leftover medication. Furthermore, only 13 patients received perioperative counseling on appropriate storage and disposal of opiates. A recent randomized control trial among 202 pediatric patients undergoing otolaryngologic or urologic procedures found that compared with providing only standard postoperative discharge instructions on opioid use, storage, and disposal, also providing a drug disposal bag significantly increased the rate of proper disposal of excess opioids by approximately 20%.4 These results suggest that a greater availability of disposal products may complement ongoing prescribing reduction efforts aimed at decreasing opioid misuse.
There are several reasons for the opioid crisis in urology, namely a culture of overprescribing.5 This may be due to:
- A historic failure to address acute pain in hospitalized patients, leading to the American Pain Society suggesting that pain should be akin to the fifth vital sign.6 Subsequently, physicians became more aware of their patients’ pain and were expected to treat their pain leading to an environment where liberal use of narcotics was tolerated.
- Over the past two decades, reimbursement, specifically through the Center for Medicare and Medicaid Services (CMS), has been linked to Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey. In the questionnaire, there were three questions dedicated to how well the patient’s pain was managed. These additional measures emphasizing the importance of pain management likely influenced the number of narcotics prescribed at discharge in order to maintain positive survey scores.
- Because narcotics must be prescribed via a hand-written prescription and obtaining additional pain medication is inconvenient for both patient and physician, providers may be more likely to overprescribe narcotics at discharge “just in case”.

The American Urological Association’s (AUA) Quality Improvement Summit on Opioid Stewardship in Urology
The AUA Quality Improvement Summit took place at AUA headquarters in December 2018 and was divided into four sessions:- Session 1: Physician-led Multicomponent Interventions in Opioid Stewardship. Dr. Richard Barth discussed procedure-specific opioid prescribing guidelines, Dr. Jonah Stulberg discussed opioid reclamation efforts, and Dr. Jim Dupree presented the Michigan MUSIC initiative on opioid stewardship.
- Session 2: Understanding Post-operative Pain. Dr. Brooke Chidgey discussed the pathophysiology of post-operative pain, Dr. Meghan Sperandeo-Fruge highlighted complementary alternative medicine pain management strategies, and Dr. Margaret Rukstalis discussed cognitive behavioral therapy and other non-pharmacologic approaches to pain management. In a sub-session discussing challenging cases in opioid management, Dr. Vernon Pais discussed the impact on prescription opioid use in patients with kidney stones, Dr. Matthew Nielsen highlighted the University of North Carolina Health Care System’s opioid stewardship program, and Dr. Benjamin Davies discussed his initiative of no opioids after a robotic prostatectomy.
- Session 3: High-Risk Patients and Expectations. Dr. Behfar Ehdaie presented on the expectation setting for opioid prescribing, Dr. Margaret Rukstalis discussed a surgeon’s role in the management of opioid misuse disorders, and Dr. Brooke Chidgey discussed the role of pain specialists for managing high-risk patients.
- Session 4: Policy and Outreach. Dr. Jennifer Waljee discussed opioid education and outreach, Dr. Scott Winiecki presented on opioid prescribing and the FDA safe use initiative, and Dr. Gregory Murphy completed the program discussing policy change and legislature to address the opioid crisis.
The full resources and slides for the AUA Quality Improvement Summit are available at: https://www.auanet.org/education/educational-calendar/quality-improvement-summit
Non-Opioid Measures for Pain Control
Data from Sweden suggests that opioid dependence may be specific to the U.S. Among 25,703 men in the National Prostate Cancer Register of Sweden who underwent radical prostatectomy, 16,368 men (64%) filled an opioid prescription during the 13 months before or after surgery.8 The use of strong opioids increased with time and the use of weak opioids decreased. There were 1.9% of men that had opioid prescriptions during the baseline period, followed by a spike to 59% around the time surgery, which sharply decreased by two months postoperatively. However, thereafter the proportion of men with opioid prescriptions remained slightly higher at 2.2% compared to the baseline before radical prostatectomy. Of chronic late users, 57% were previous users and 43% were new chronic users. Higher cancer risk category, greater comorbidity, unmarried status and low educational level were associated with the risk of new chronic opioid use. Although more than half of male Swedish patients filled an opioid prescription after radical prostatectomy, less than 1% of men became chronic opioid users.Professor Benjamin Davies from the University of Pittsburgh has been a thought leader and advocate for minimizing opioid prescriptions among patients undergoing urologic procedures, namely advocating for the “No Opioid Robotic Radical Prostatectomy”.9,10 This protocol is as follows:
- Pre-operative: Oral neurontin, acetaminophen, +/- celebrex
- Quadratus lumborum block (ropivicaine, decadron, precedex)
- Intraoperative: separate infusions of propofol, ketamine, and precede
- Post-operative: Toradol 15 mg IV PRN while in the hospital
- Tylenol and Motrin for 48 hours
Prospective Initiatives
In an effort to evaluate the effect of opioid reduction after radical prostatectomy on post-discharge opioid prescribing, use, and disposal, the ORIOLES trial was designed as a prospective, non-randomized, pre-post interventional trial.12 An evidence-based intervention included a discharge sheet, nursing education, and standardized prescribing guideline; the primary outcome was total oral morphine equivalents used after surgery. Secondary outcomes included opioid prescribing, opioid disposal, need for additional opioid medication, and presence of incisional/post-surgical abdominal pain beyond 30-days. There were 214 men in the pre-intervention arm and 229 men in the post-intervention arm with 100% follow-up. The intervention reduced post-discharge opioid prescribing from 224.3 mg to 120.3 mg (p=0.01), reduced opioid use from 52.1mg to 38.3mg (p<0.01), and increased opioid disposal by 13.5% (p<0.01). Greater post-discharge opioid use was associated with greater prescribing of opioids at discharge, higher body mass index, and use of opioid medication prior to surgery.From this prospective initiative, the authors demonstrate that a simple, three-component opioid reduction intervention was able to reduce opioid prescribing, reduce opioid use, and increase opioid disposal at 30-days after radical prostatectomy. Importantly, this prescribing guideline met the needs of 84% of patients, while only 2.2% of patients required additional opioid medication for pain. Furthermore, an impressive one-third of patients used no opioid pain medication after discharge.
Investigators from the Mount Sinai School of Medicine have also recently assessed the effect of implementing a nonopioid protocol for patients undergoing robotic-assisted radical cystectomy with extracorporeal urinary diversion.13 Among 52 patients undergoing surgery, patients received a multimodal pain management protocol, including a combination of nonopioid pain medications and regional anesthesia. These patients were compared to 41 patients undergoing robotic cystectomy prior to the implementation of the nonopioid protocol. In this study, the authors found that patients on the nonopioid protocol received a much lower dose of postoperative morphine milligram equivalents (2.5 vs. 44, p < 0.001), with no difference in pain scores. In the non-opioid protocol patients, the median time to regular diet was significantly shorter (4 days vs. 5 days, p = 0.002), and the length of stay was two days shorter compared to the control group (5 days vs. 7days, p < 0.001).
Conclusions
The urologic community has by no means been spared by the current opioid epidemic across the U.S. Several studies in both the adult and pediatric settings have demonstrated overprescribing measures with little to no counseling or options for appropriate disposal of opioids. Several measures are now in place to solve this problem5, including (i) greater utilization and implementation of Prescription Drug Monitoring Programs (PDMPs) to provide alerts to providers to patients who may be filling opiate prescriptions with multiple providers; (ii) CMS has removed the three questions from the HCAHPS survey related to pain control, effective January 2018; (iii) increased utilization of Enhanced Recovery After Surgery (ERAS) pathways as a measure for decreasing intra- and post-operative use of opioids; (iv) each of the 50 states have passed legislation to make readily available naloxone, which rapidly reverses the effects of opioids in the overdose setting; (v) the creation of procedure-specific guidelines for discharge opioid recommendations. For example, a Johns Hopkins expert panel assessing 20 common surgical procedures suggest that the ideal range of oxycodone 5-mg tablets prescribed to opioid naïve patients at discharge is 0-1014; (vi) the DEA sponsored “National Rx Takeback” initiative, providing collection sites (primarily pharmacies) for returning opioids. Certainly, the current opioid epidemic is multifactorial. However, judicious prescribing of opioids amongst the urology community is one actionable item that will make a difference for the betterment of our patients.Published Date: December 2019