Introduction
The treatment landscape of metastatic castrate-resistant prostate cancer (mCRPC) has long been dominated by androgen receptor pathway inhibitors (ARPIs) and chemotherapeutic agents. There are currently, however, two radioligands that are United States Food and Drug Administration (FDA)-approved for the treatment of mCRPC patients:
- Radium-223 (Xofigo®) for mCRPC patients with symptomatic bone metastases and no known visceral metastatic disease (2013)1
- Lutetium 177 vipivotide tetraxetan (PLUVICTO®) for prostate-specific membrane antigen (PSMA)-positive mCRPC patients who had previously been treated with an ARPI and taxane-based chemotherapy
In this Center of Excellence article, we will discuss the existing evidence informing the regulatory approvals of radium-223 and Lu-PSMA, as well as emerging radioligands, including combination approaches, in the mCRPC treatment space.
Radium-223
Radium-223 is a targeted alpha emitter that selectively binds to areas of increased bone turnover in bone metastases and emits high-energy alpha particles of short range (<100 μm). Radium-223 acts as a bone-seeking calcium mimetic and binds into newly formed bone stroma, especially within the microenvironment of osteoblastic or sclerotic metastases. Double-stranded DNA breaks result secondary to the high-energy alpha-particle radiation. This results in a potent and highly localized cytotoxic effect in the target areas. Furthermore, the short path of the alpha particles also theoretically minimizes the toxic effects on adjacent healthy tissue, including the bone marrow.3
ALSYMPCAThe ALSYMPCA trial was a phase III randomized controlled trial (RCT) of 921 mCRPC patients randomized 2:1 to receive six IV injections of radium-223 (50 kBq/Kg) or matching placebo. All patients received additional best standard of care. Of note, prior docetaxel treatment was permitted (57% of included patients). Patients receiving radium-223 demonstrated significantly improved median overall survival (14 versus 11.2 months; HR: 0.70, 95% CI: 0.55 – 0.88):

Use of radium-223 was further associated with significantly prolonged time to a first symptomatic skeletal event (15.6 versus 9.8 months, p<0.001) and a significantly higher percentage of radium-223-treated patients achieved clinically meaningful improvements in quality-of-life outcomes (i.e., ≥10-point improvement in the FACT-P total score; 25% versus 16%, p = 0.02). Grade 3–4 adverse events were observed less frequently in the treatment arm (56% versus 62%). Notable grade ≥3 adverse events included anemia (13% in both arms) and thrombocytopenia (6% versus 2%). Febrile neutropenia was observed in only one patient in each arm.3
REASSUREReal-world prospective evaluation of the outcomes of radium-223-treated patients is of utmost importance given the known external validation (i.e., generalizability) limitations of RCTs to real-world practice. REASSURE (NCT02141438) is an ongoing, global multicenter, prospective, observational, single-arm study of radium-223 use in clinical practice between 2014 and 2017 (n = 1,465):

Results of an interim analysis, at a medium follow-up of 11.5 months, were published in 2023.4 All six planned cycles of radium-223 treatment were completed by 59% of patients. Treatment-emergent serious adverse events occurred in 21% of patients, and drug-related treatment-emergent adverse effects of any grade in 35% of patients. The most common drug-related treatment-emergent adverse events were diarrhea (11%), nausea (9%), and anemia (8%). Drug-related adverse events leading to radium-223 discontinuation and death occurred in 6% and 1% of patients, respectively. In the 6 months following completion of radium-223 therapy, 15% of patients had grade 3–4 hematologic toxicities (anemia: 12%, thrombocytopenia: 4%, neutropenia: 1%, pancytopenia <1%).
The median overall survival was 15.6 months (95% CI: 14.6–16.5) and disease progression was the main cause of death (77% of deaths). Overall, 32% of patients received ≥1 subsequent systemic life-prolonging anticancer therapy (48% never received taxane therapy):
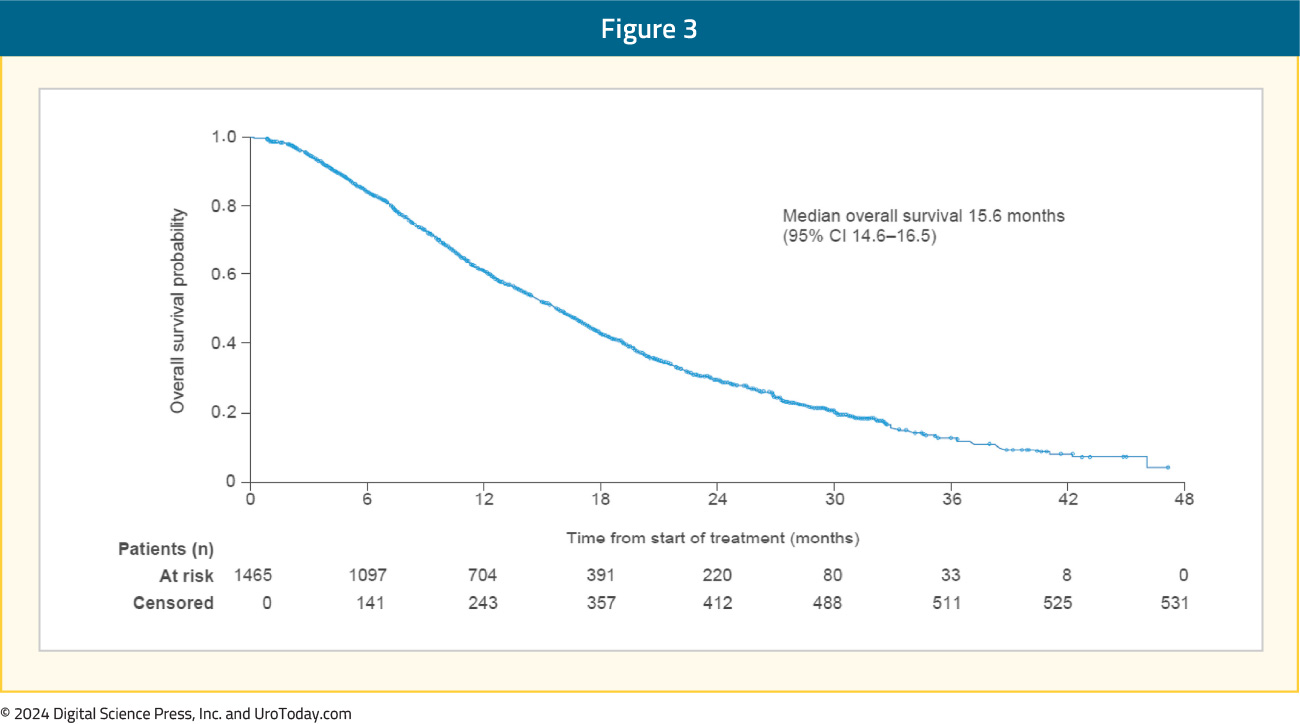
During treatment, 55% of patients had a clinically meaningful pain response (≥2 points improvement in the “pain at its worst” item of the BPI-SF scale), and fractures occurred in 5% of patients. Twenty-one patients developed a second primary malignancy during or after receipt of radium-223. Notably, 16/21 patients had received prior (n = 15) or concurrent radiotherapy (n = 1).
Real-World UtilizationThe results of a real-world analysis of the Komodo Research Dataset, an administrative claims dataset of >140 million individuals from over 150 private insurance providers in the United States (commercial and Medicare), were presented at ASCO GU 2024. This study included 1,376 radium-223 users. The prescribing physician was most commonly a medical oncologist (52%). Radium-223 was most commonly prescribed in the 2nd (35%) and 3rd line (25%) settings. Of note, nearly half of the patients (49%) had visceral metastases, and ≥5 cycles of radium-223 were completed by 46% of patients in the cohort (54% of those surviving ≥6 months). Radium-223 was utilized as a combination therapy by 26% of the overall population, predominately with enzalutamide:

The results of a Spanish real-world study from seven Galician hospitals of 146 mCRPC patients receiving radium-223 in the ≥2nd line setting were presented at ASCO GU 2023. At a median follow-up of 9.6 months, the median overall survival was 12 months (95% CI: 9.7–16). The median overall survival was 18 months for those receiving radium-223 in the 2nd line setting and 9.4 months in the 3rd line setting:6

177Lutetium–PSMA-617
PSMA is a transmembrane protein expressed in all forms of prostate tissue, including carcinoma. The PSMA gene is located on the short arm of chromosome 11 in a region that is not commonly deleted in prostate cancer, thus making it highly prevalent in all forms of prostate cancer, including mCRPC. Importantly, PSMA is relatively poorly expressed in other organs (apart from salivary), therefore PSMA-targeted theranostic treatment allows for relatively specific targeted therapy. PSMA does have an internalization signal that allows for internalization of the extracellular component into an endosomal compartment.7 This has allowed for the combination of PSMA-617 and the beta-emitter lutetium to deliver targeted ß-particle radiation to PSMA-expressing cells and the surrounding microenvironment.
There are two major trials that have led to the regulatory approval of 177Lu-PSMA-617 in the post-taxane and ARPI mCRPC setting: VISION and TheraP.8,9
VISION
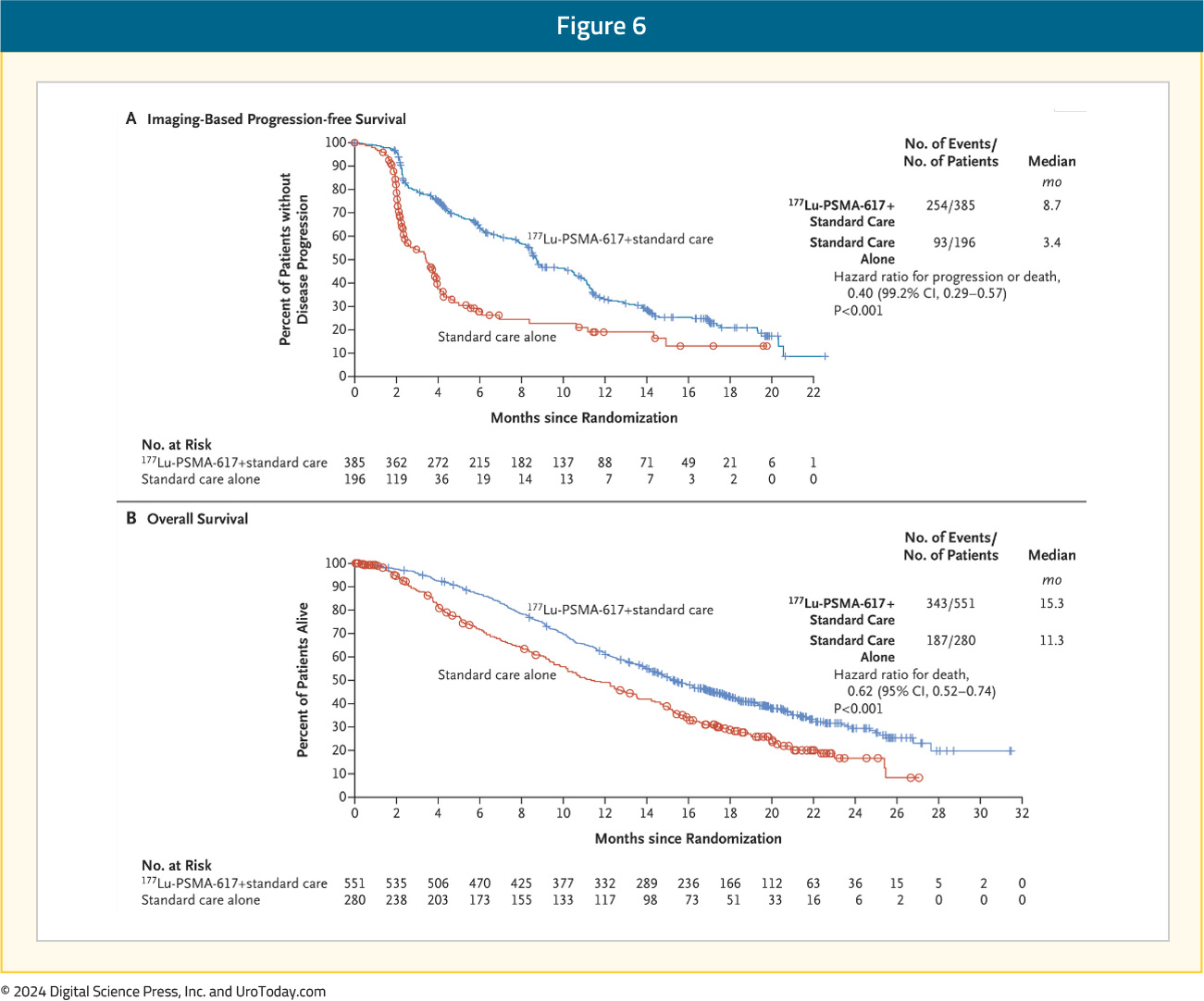
VISION is an international, open-label, phase 3 trial that evaluated 177Lu-PSMA-617 in mCRPC patients previously treated with an ARPI and 1–2 taxane regimens and who had PSMA-positive 68Ga-PSMA-PET/CT scans. Overall, 831 patients were randomly assigned in a 2:1 ratio to receive either 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 4–6 cycles) plus protocol-permitted standard care or standard care alone. At a median follow-up of 20.9 months, 177Lu-PSMA-617 plus standard of care significantly prolonged both radiographic progression-free survival (median: 8.7 versus 3.4 months; HR: 0.40, p < 0.001) and overall survival (median: 15.3 versus 11.3 months; HR: 0.62; 95% CI: 0.52 to 0.74, p < 0.001):
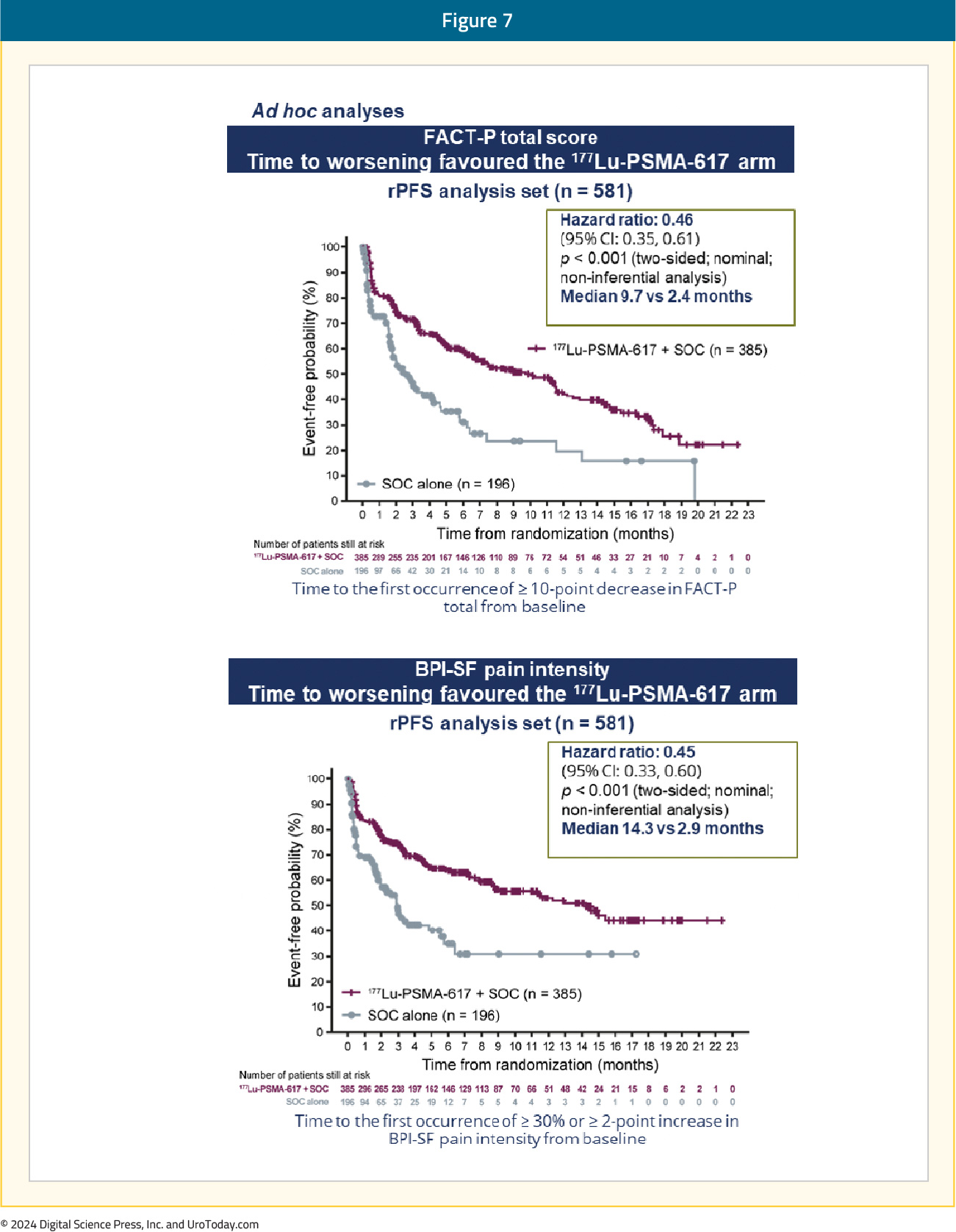
Patient reported outcomes as evaluated by the FACT-P and Brief Pain Inventory scores favored the 177Lu-PSMA-617 arm with delays in time to worsening of 7.3 and 11.4 months, respectively:
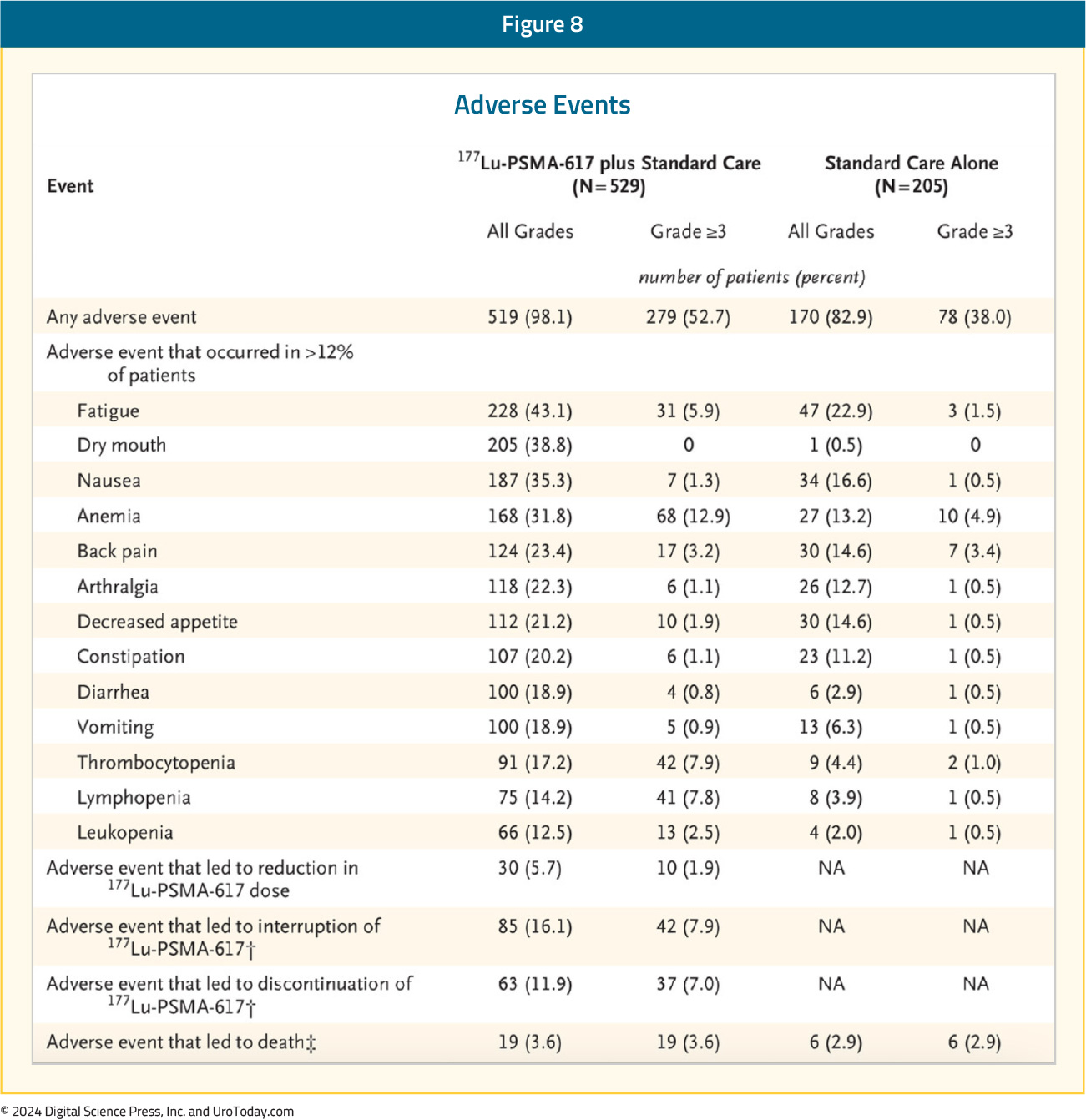
While a higher rate of grade 3–5 treatment-emergent adverse events was observed with 177Lu-PSMA-617 (28% versus 4%) at the time of initial reporting, overall therapy was well tolerated. It bears note that treatment exposure was more than three times longer in the 177Lu-PSMA-617 group than in the control group. Bone marrow toxicity with grade ≥3 anemia, leukopenia, and thrombocytopenia was observed in 13%, 2.5%, and 8% of patients in the 177Lu-PSMA-617 arm, respectively. Although 39% of 177Lu-PSMA-617 treated patients experienced any grade xerostomia, none were grade 3 or worse:
TheraP was an open label, phase II trial of 200 mCRPC men who were randomized to either 177Lu-PSMA-617 or cabazitaxel. To screen into the study, all men had both 68Ga-PSMA-11 and 18F-FDG PET/CT and were required to have high PSMA-expression (≥1 site with SUVmax ≥20) and no sites of FDG-positive/PSMA-negative disease. All patients had progressive disease with rising PSA ≥20 ng/mL after docetaxel and 91% had received prior enzalutamide or abiraterone. There were 200 patients were randomized 1:1 to 177Lu-PSMA-617 6-8 GBq every 6 weeks for up to 6 cycles of therapy or cabazitaxel 20 mg/m2 every three weeks for up to 10 cycles:
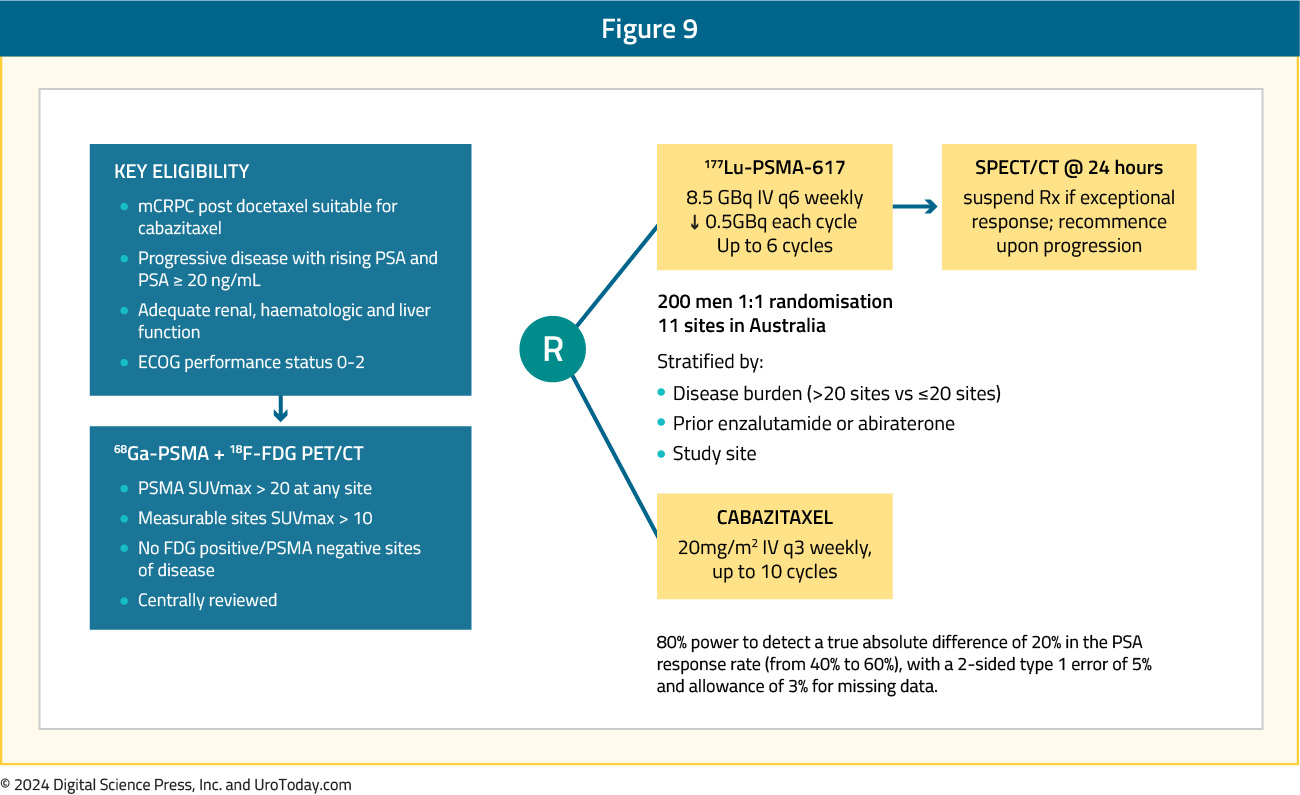
After a median follow up of 13 months, 177Lu-PSMA-617 significantly improved PSA progression-free survival compared with cabazitaxel (HR 0.63, 95% CI 0.46 to 0.86) and was associated with a much higher PSA50 response (66% vs 37%):

Grade 3–4 toxicity was observed in 54% of men on cabazitaxel compared to 35% of patients who received 177Lu-PSMA-617. Rates of thrombocytopenia, dry mouth, and dry eyes were seen more frequently in patients receiving 177Lu-PSMA-617, as expected due to normal PSMA expression in the salivary and lacrimal glands.9
An updated report of TheraP was published in The Lancet Oncology in 2024. At a median follow-up of three years, there were no significant differences in overall survival between the two arms (19.1 versus 19.6 months for 177Lu-PSMA-617 and cabazitaxel, respectively; p = 0.77):10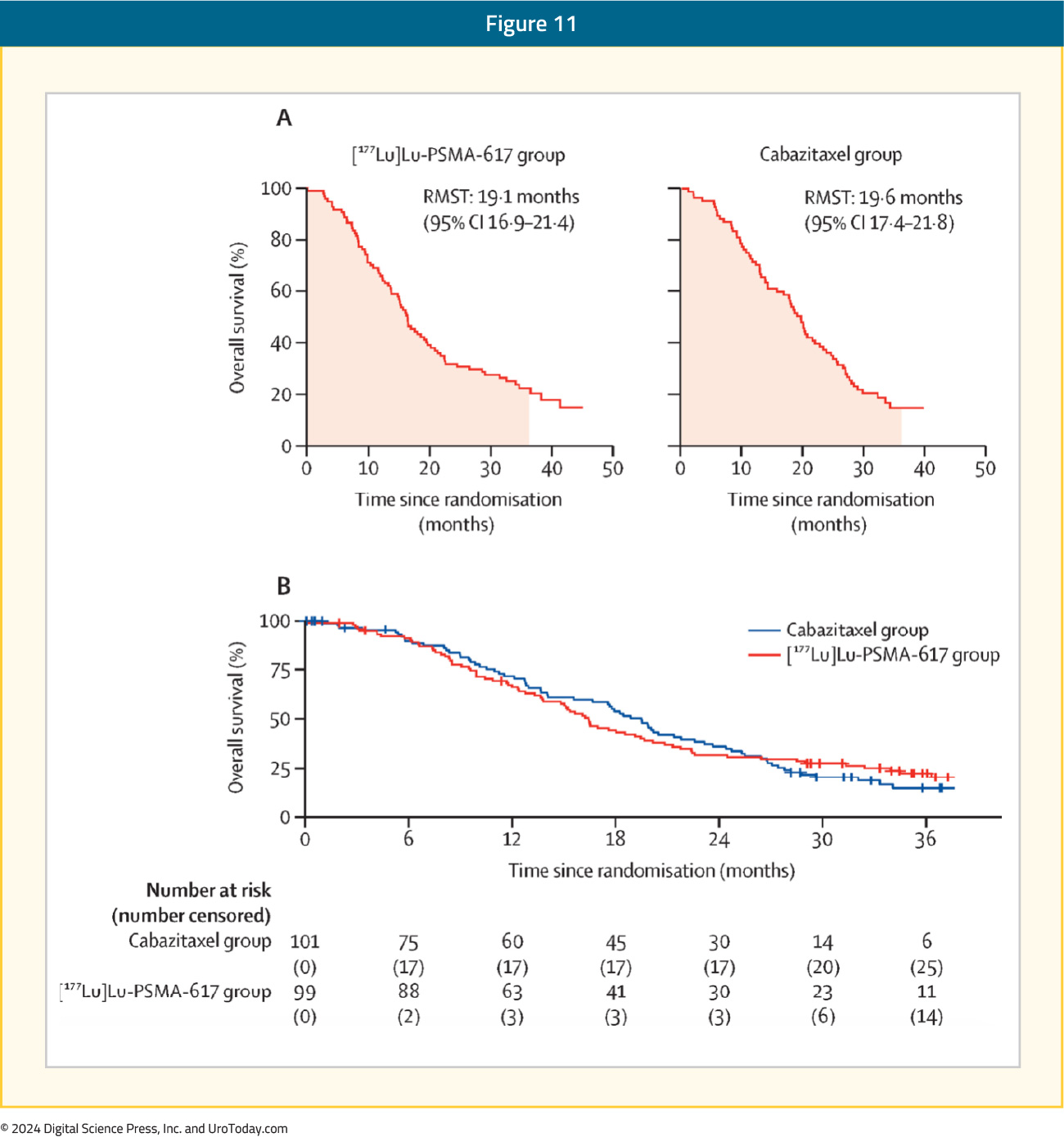
Based on the results of these trials, the current guidelines strongly recommend considering the use of 177Lu-PSMA-617 in taxane and ARPI-pre-treated mCRPC patients who have evidence of PSMA-expressing metastatic lesions as per either 68Ga or 18F-DCFPyL PSMA-PET/CT.
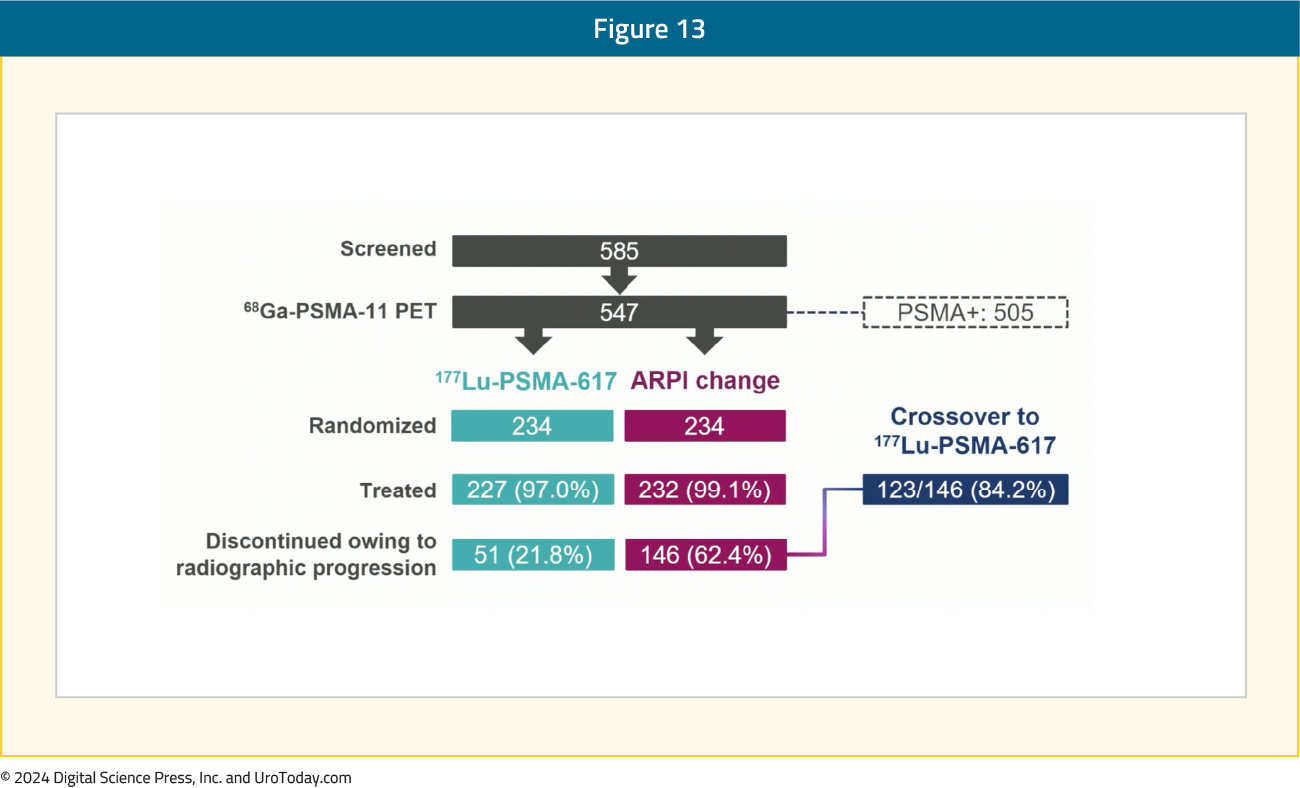
Presented at ESMO 2023, PSMAfore was the first 177Lu-PSMA-617 trial to assess radioligand therapy in the docetaxel-naïve mCRPC population. Eligible adults for PSMAfore had mCRPC, were candidates for ARPI change after one progression on prior ARPI, and had ≥1 PSMA positive lesions and no exclusionary PSMA negative lesions by 68Ga-PSMA-11 PET/CT. Randomization was 1:1 to open-label 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 6 cycles) or ARPI change (abiraterone or enzalutamide). Importantly, patients randomized to ARPI could crossover to 177Lu-PSMA-617 following centrally reviewed radiographic progression. The trial design for PSMAfore is as follows:

Overall, there were 468 patients randomized. As follows is the patient disposition at the second interim OS analysis, noting that 84.2% of patients that had an rPFS event in the ARPI change subsequently went on to receive crossover 177Lu-PSMA-617:
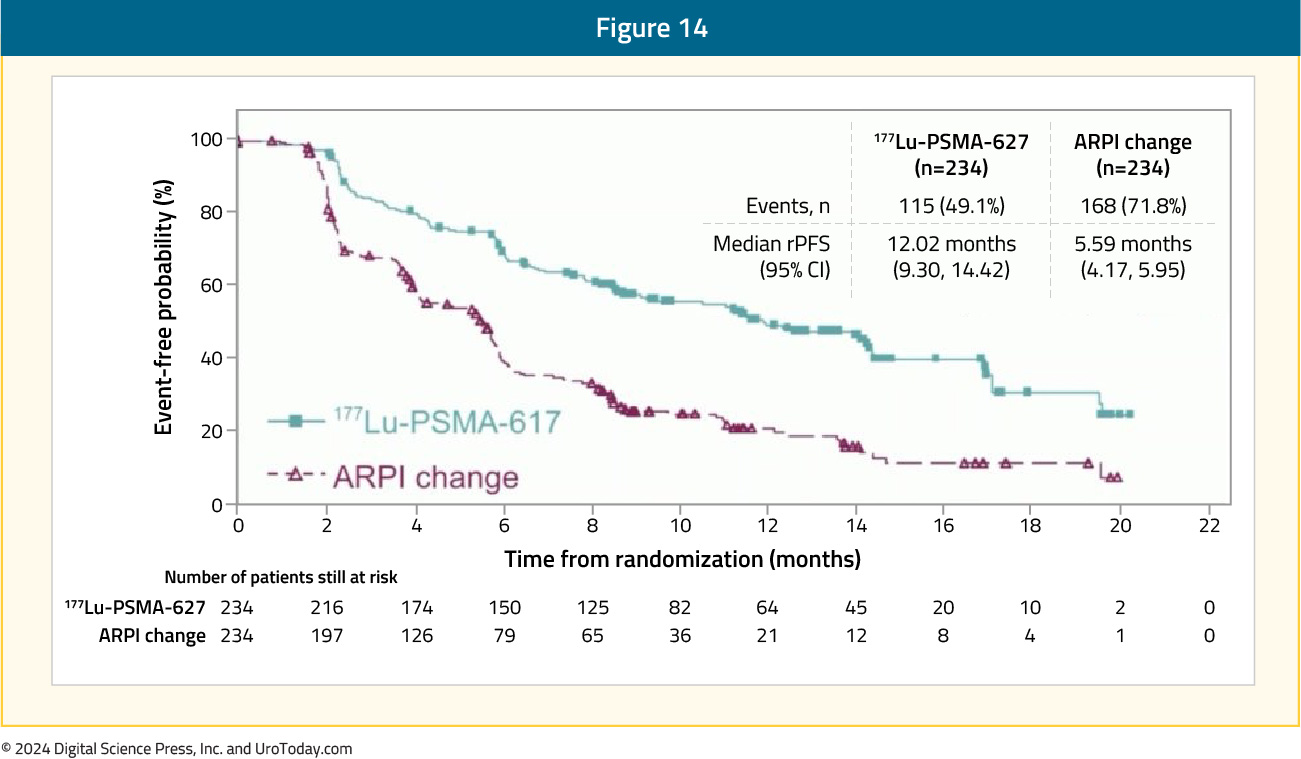
At the primary analysis (median follow-up, 7.3 months; n = 467), the primary endpoint of rPFS was met (HR 0.41, 95% CI 0.29 to 0.56), which was similar at the second interim analysis (HR 0.43, 95% CI 0.33 to 0.54):
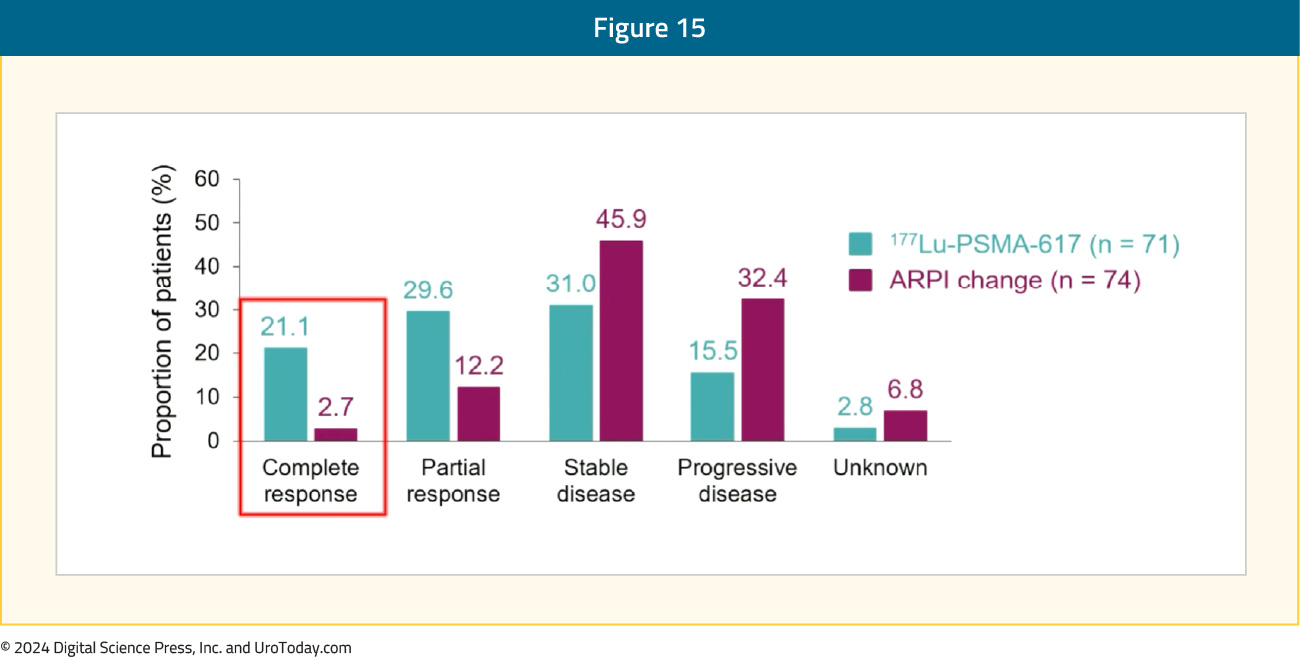
Among men with measurable disease, the objective response rate was 50.7% for 177Lu-PSMA-617 versus 14.9% for ARPI change. The median duration of response was 13.63 months (95% CI 11.56 – NE) for 177Lu-PSMA-617 versus 10.05 months (95% CI 4.63 – NE) for ARPI change. As follows is a summary of the radiographic response rates, highlighting that patients in the 177Lu-PSMA-617 arm had a complete response rate of 21.1% versus 2.7% in the ARPI change arm:
Patients receiving 177Lu-PSMA-617 had a confirmed PSA decrease rate >=50% in 57.6% of patients compared to 20.4% of patients in the ARPI change arm. Time to symptomatic skeletal events also favored the 177Lu-PSMA-617 arm (HR 0.35, 95% CI 0.22 to 0.57). Time to composite health related quality of life or pain worsening as summarized by the FACT-P total score (HR 0.59, 95% CI 0.47 to 0.72) and BPI-SF pain intensity scale (HR 0.69, 95% CI 0.56 to 0.85), respectively, also favored 177Lu-PSMA-617. Additionally, at the second interim analysis (45.1% of target deaths), 123/146 (84.2%) patients with reviewed radiographic progression who discontinued ARPI crossed over to receive 177Lu-PSMA-617. In the pre-specified cross-over adjusted analysis, there was a trend favoring 177Lu-PSMA-617, but no statistical difference in OS between the groups:

In the unadjusted, intention to treat analysis for OS, the HR was 1.16 (95% CI 0.83 to1.64). For 177Lu-PSMA-617 vs ARPI change, the incidence of grade ≥3 adverse events was 33.9% (most commonly anemia and dry mouth) versus 43.1%, serious adverse events 20.3% vs 28.0%, and adverse events leading to discontinuation 5.7% vs 5.2%, respectively.
RALU: 177Lu-PSMA Following Radium-223The radium lutetium (RALU) study was a retrospective, multicenter study evaluating the safety of 177Lu-PSMA-617 in 49 German mCRPC patients previously treated with radium-223. Overall, 92% of patients had received ≥1 line of taxane-based chemotherapy, and 63% had received ≥3 life-prolonging therapies (docetaxel, cabazitaxel, abiraterone, enzalutamide, and radium-223):

The median 177Lu-PSMA-617 therapy duration was 4.9 months, and the median time from the last radium-223 injection to the first 177Lu-PSMA-617 dose was 9.3 months (range: 1–42). Grade 3–4 treatment-emergent adverse events were experienced by 41% of patients, grade 3–4 anemia and thrombocytopenia occurred in 18% and 2% of patients, respectively, grade 1–2 xerostomia occurred in 27% of patients; none had grade 3–4 dry mouth. There were no grade 5 toxicities that occurred.
The median overall survival was 12.6 months from the 1st dose of 177Lu-PSMA (95% CI: 8.8–16.1). During 177Lu-PSMA, 29% of patients achieved a PSA50 response:11

Actinium-225
Similar to radium-223, actinium-225 (225Ac) is an alpha emitter, which delivers significantly higher energy levels compared to β-emitters (4–9 MeV vs. 0.1–2.2 MeV). Combined with shorter path lengths, this results in a higher linear energy transfer and a greater probability of generating DNA double-strand breaks on interaction with cell nuclei. This makes them more effective to tumor cellular killing with significantly fewer DNA hits.12
WARMTH ActWARMTH Act is an international cohort study of 488 mCRPC patients who received 225Ac-PSMA radioligand therapy following disease progression with earlier line agents.13 The mean patient age was 68 years, and the median baseline PSA level was 170 ng/ml. PSMA-avidity in the mCRPC lesions, and thus treatment eligibility, was determined primarily using PET (95%). This was a heavily pre-treated population with 53% of patients having received ≥3 prior lines of therapy for mCRPC. Indeed, 91% of patients received 225Ac-PSMA as a last line therapy option because they had exhausted or were ineligible for other treatment options. Prior therapies included:
- Docetaxel: 66%
- Cabazitaxel: 21%
- Abiraterone: 39%
- Enzalutamide: 39%
- 177Lu-PSMA: 32%
- Radium-223: 4$
Patients received a median of two (IQR:2–4) treatment cycles, and 57% of patients completed their 225Ac-PSMA radioligand therapy as planned. Reasons for interruption included favorable response to treatment (n = 84), disease progression (n = 24), logistical issues with supply availability (n = 34), xerostomia (n = 11), and unstable clinical condition (n = 8).
Any PSA decline after ≥1 cycle of 225Ac-PSMA was observed in 73% of patients, with a PSA50 response observed in 57% of patients. 33% of patients had a radiographic response based on 68Ga PSMA-PET/CT findings of minimal residual disease. The administered activity of 225Ac-PSMA radioligand therapy was reduced for salivary gland protection in these patients:

The median follow-up duration was 9 months, and the median progression-free survival was 9 months (95% CI: 5–17.5). At the time of data collection, 63% of patients had died, with all deaths secondary to mCRPC and its complications. The median overall survival was 15.5 months (95% CI: 13.4–18.3). Patients who did not achieve a PSA50 response, those with liver or peritoneal metastases, and anemia at baseline had worse survival outcomes with 225Ac-PSMA on multivariable analysis. No treatment-related death was recorded. Notably, only 10% of patients who died received another form of treatment after disease progression.
The most common adverse event was xerostomia. This was observed in 68% of patients after the 1st cycle of treatment and in ≥86% of patients who received ≥2 cycles; however, only 2% of patients discontinued treatment secondary to xerostomia. After 225Ac-PSMA radioligand therapy, any grade of anemia was observed in 81%, leukopenia in 44%, and thrombocytopenia in 54%. Grade ≥3 anemia, leukopenia, and thrombocytopenia were observed in 13%, 4%, and 7% of patients, respectively. It bears note, however, that a significant proportion of patients had baseline bone marrow dysfunction.
Select Radioligand Combination Therapies
ENZA-p: 177Lu-PSMA-617 + Enzalutamide
One example of a radioligand combinatory approach that allows for the targeting of cells without PSMA expression is the combination of 177Lu-PSMA-617 + enzalutamide. There is a close relationship between the androgen and PSMA receptors, with upregulation of PSMA expression in response to androgen blockade observed in mCRPC. This increased PSMA expression is associated with poor survival in CRPC patients receiving ARPIs. Increased PSMA expression increases dsDNA breaks and cell death with 177Lu-PSMA-617 therapy, increasing the depth of treatment response. This reduces/eliminates the high PSMA expressing clonal population, leaving a low PSMA expression clonal population more likely to respond to ARPIs. The ENZA-p trial investigators hypothesized that using both treatments together may lead to potentially deeper and longer patient responses, compared to ARPI monotherapy.
ENZA-p (ANZUP 1901) was an open label, randomized phase 2 trial across 15 centers in Australia of 162 mCRPC patients who had not previously received a taxane or an ARPI in the mCRPC setting, had 68Ga-PSMA-PET/CT-positive disease, and ≥2 risk factors for early progression on enzalutamide. These patients underwent 1:1 randomization to enzalutamide +/- 177Lu-PSMA-617:
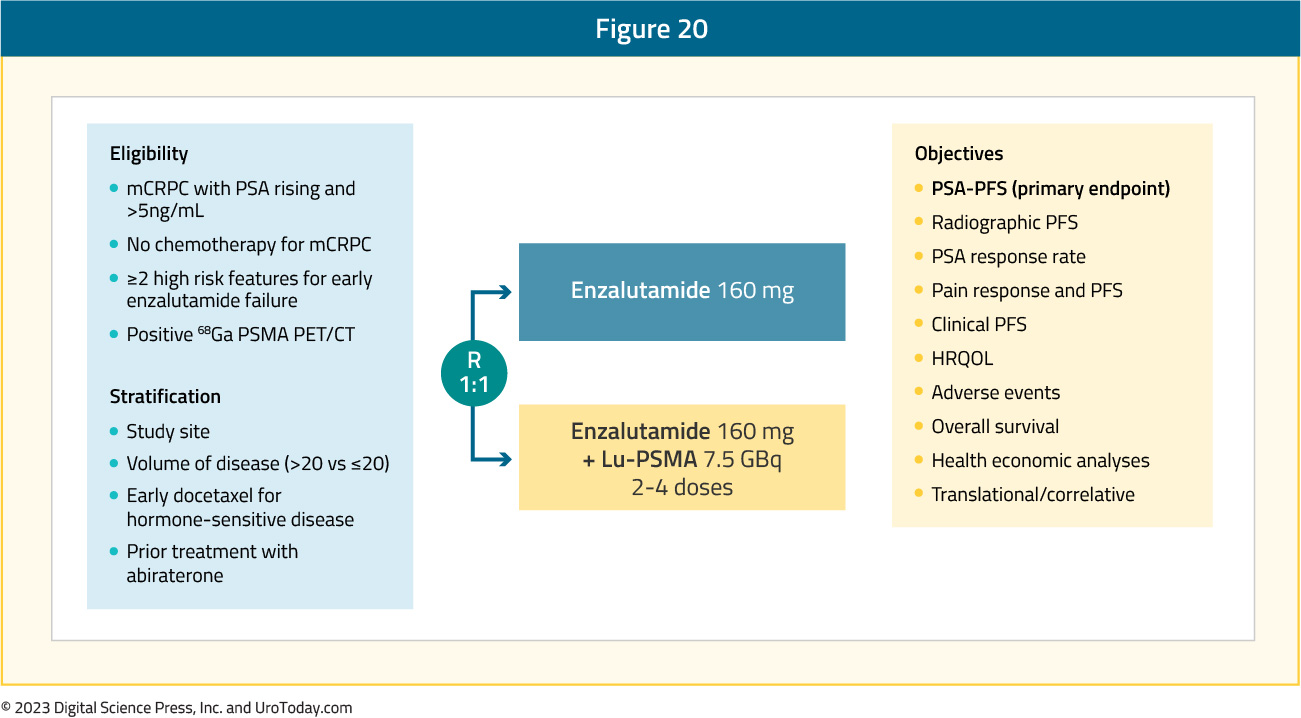
Of 220 screened patients, 160 met the eligibility criteria and were randomized (enzalutamide + Lu-PSMA, n = 83; enzalutamide, n = 79). The PSMA PET imaging screen failure rate was 18%. In the combination arm, 81% of patients received all 4 doses of 177Lu-PSMA-617, with a median follow-up was 20 months.
This trial met its primary endpoint, with the addition of 177Lu-PSMA-617 to enzalutamide improving PSA progression-free survival from 7.8 to 13 months (HR: 0.43, 95% CI: 0.29–0.63, p < 0.001). rPFS (data maturity: 60%) also favored the combination arm (median: 16 versus 12 months; HR: 0.67, 95% CI: 0.44 – 1.01).
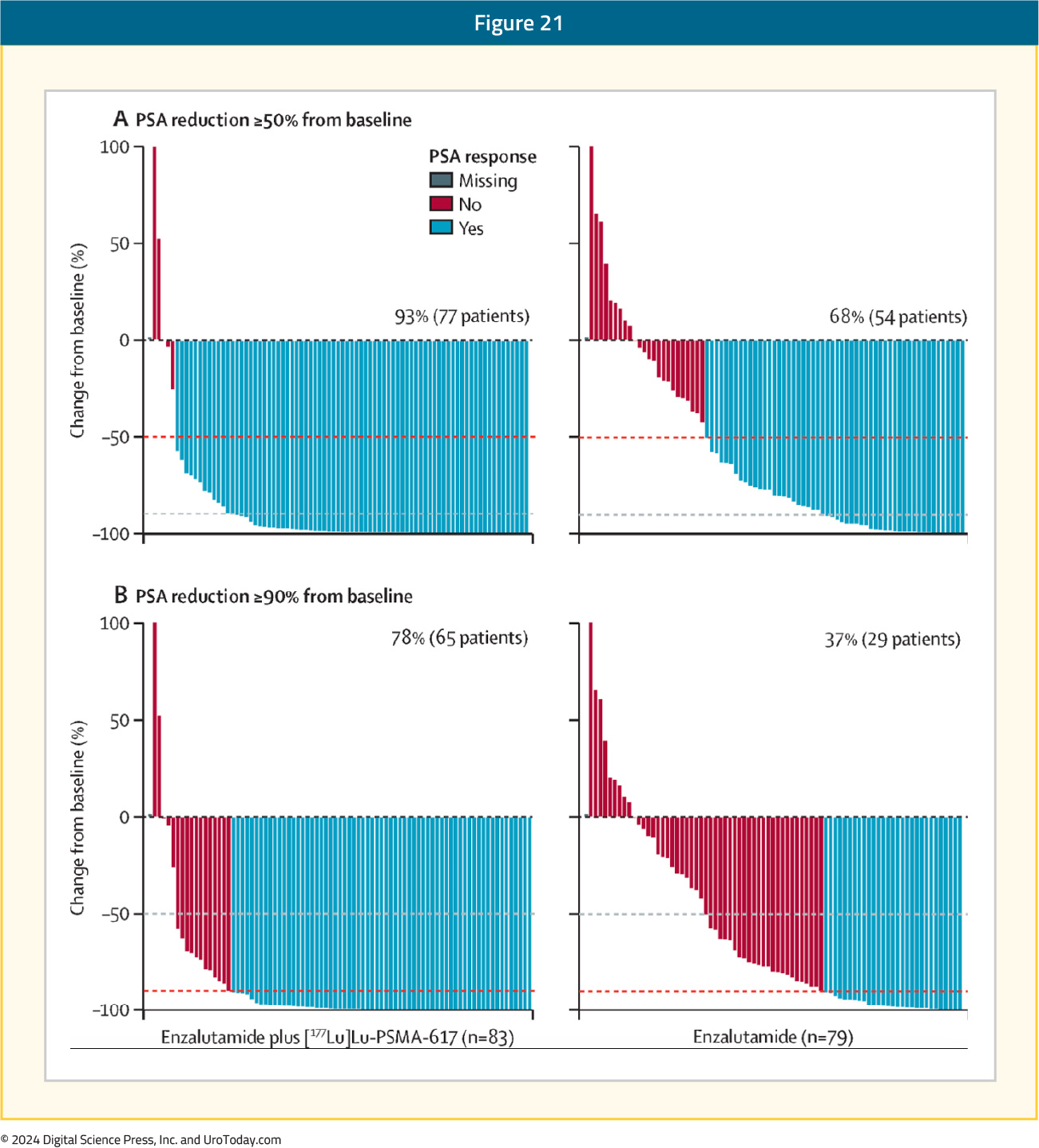
A PSA50 response was observed in 93% of patients in the 177Lu-PSMA-617 + enzalutamide arm, compared to 68% of enzalutamide-treated patients. The corresponding PSA90 responses were 78% and 37%, respectively:
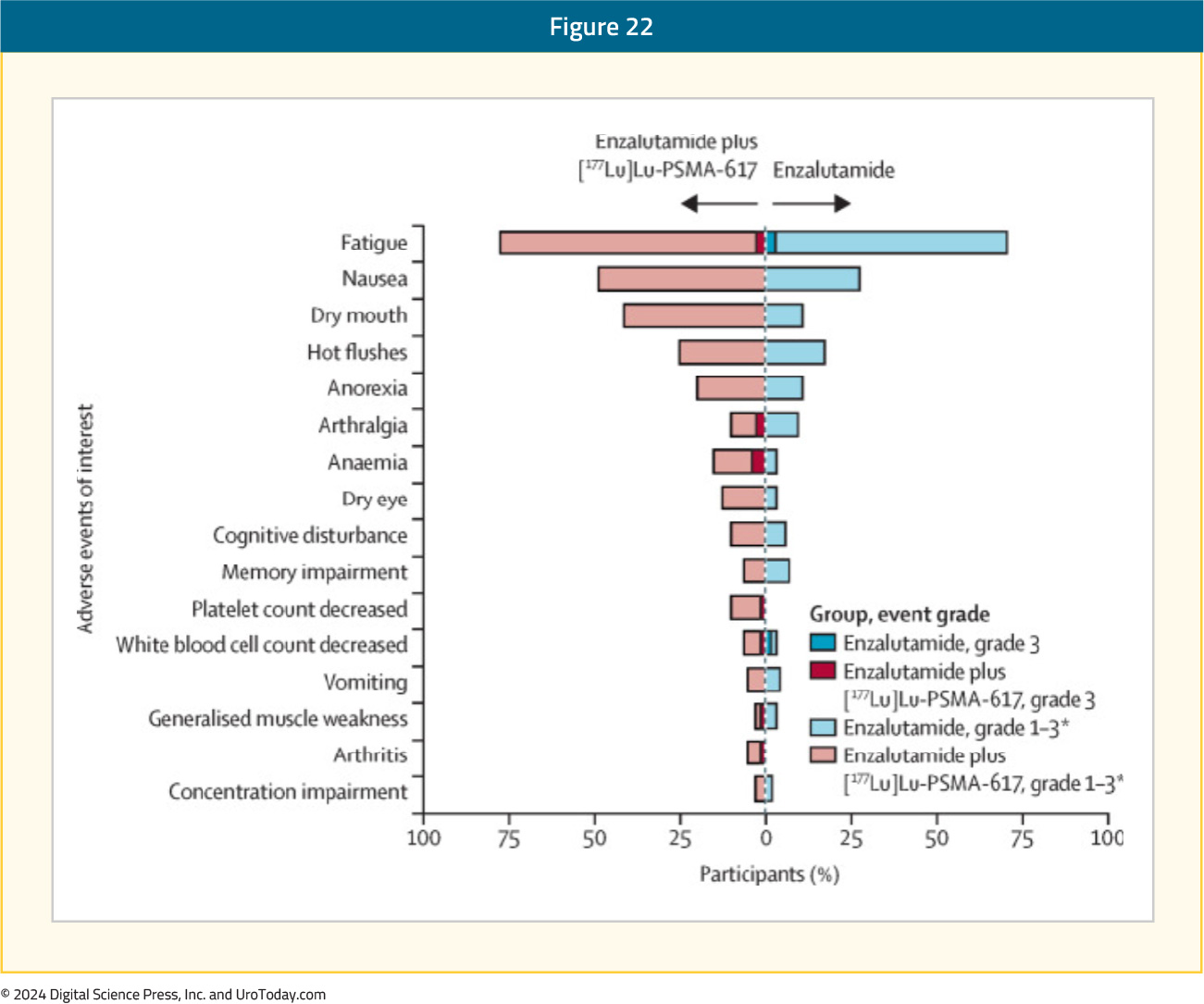
Grade 3–5 adverse events were observed in 40% and 41% of patients in the intervention and control arms, respectively. Grade 3 events that only occurred in the intervention arm were anemia (4%) and decreased platelet count (1%):14
Given that radium-223 induces DNA double strand breaks and PARP inhibitors interfere with DNA repair mechanisms, it has been hypothesized that these two agents may have synergistic mechanisms of action. COMRADE is a phase 1/2 trial evaluating the combination of olaparib and radium-223. This trial enrolled 12 patients, all of whom had received a prior ARPI, and 25% had received prior docetaxel. No dose limiting toxicities were observed. The recommended phase 2 dose was determined to be olaparib 200 mg orally twice daily with radium-223. The most common treatment-related adverse events were fatigue (92%) and anemia (58%). The 6-months rPFS was 58%, and the 12-months overall survival was 56%:15

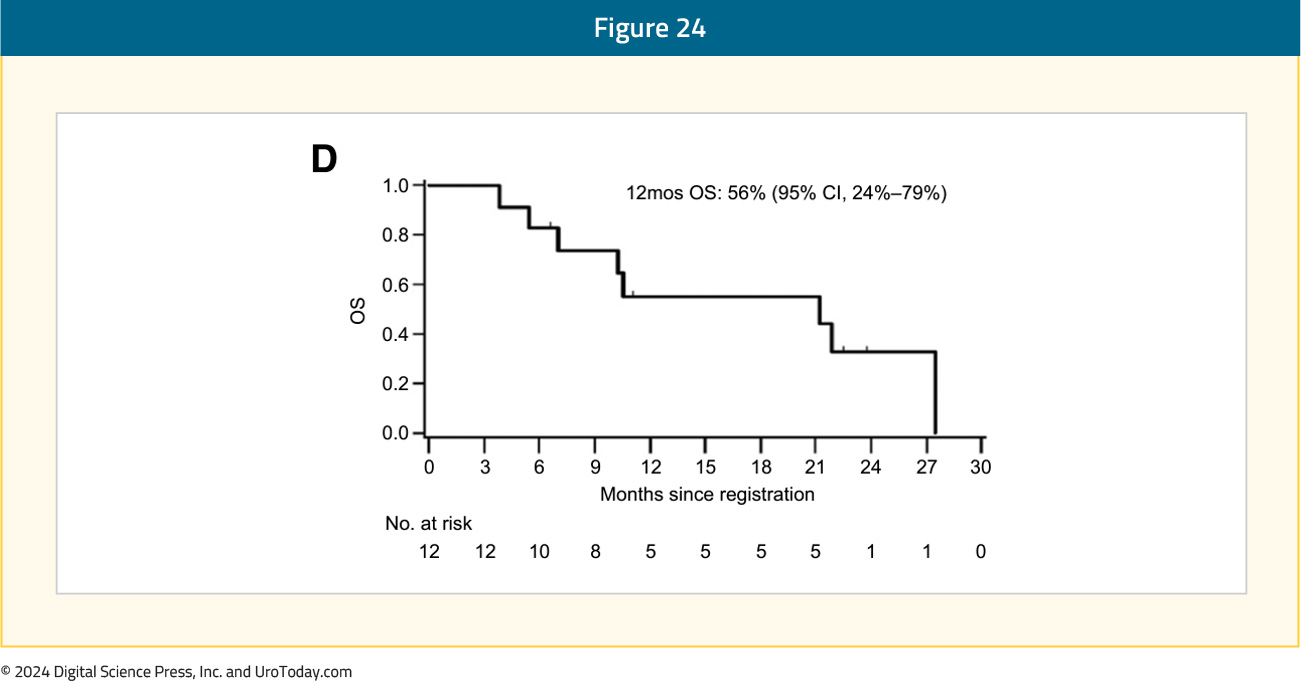
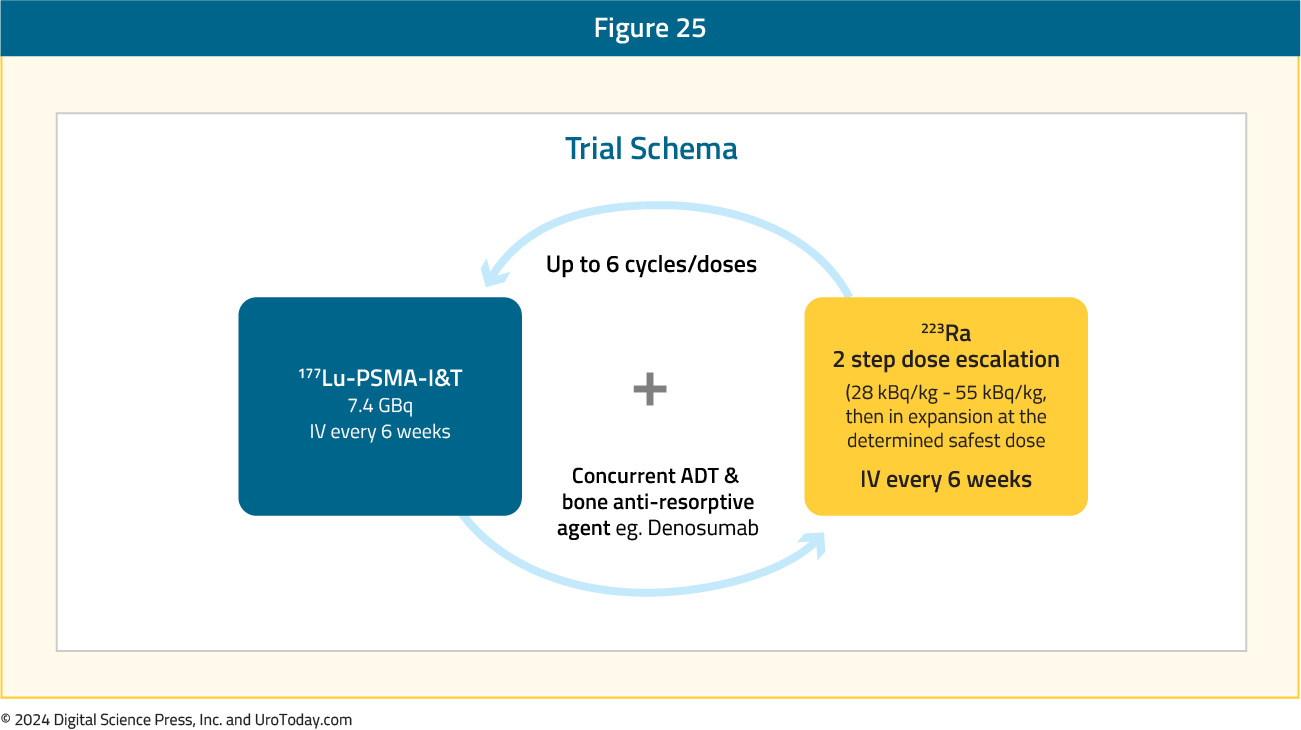
AlphaBet is an ongoing phase I/II trial evaluating the combination of radium-223 + Lu-177 PSMA-I&T in mCRPC patients. In a pre-clinical LNCaP xenograft model, this combination, compared to single agent therapy, was associated with improved PSA response.16
In AlphaBet, 36 mCRPC patients will receive up to 6 cycles of 177Lu-PSMA-I&T 7.4 GBq intravenously every 6 weeks + 223Ra in a two-step dose escalation (28 kBq/kg – 55 kBq/kg, then in expansion at the determined safest dose) intravenously every 6 weeks. The primary study objectives are to establish the ‘safe dose’ and to evaluate the anti-tumor activity. Secondary outcomes include side effects, survival outcomes, and patient-reported outcomes:
LuCAB is a phase I/II study of cabazitaxel in combination with 177Lu-PSMA-617 in mCRPC patients. The addition of cabazitaxel is hypothesized to address potential mechanisms of resistance to 177Lu-PSMA-617:
- Underlying PSMA-negative disease
- Tumor microenvironment related sensitivity (low sensitivity with hepatic/visceral metastases, high sensitivity with nodal disease)
- Micrometastatic disease not receiving adequate radiation from 177Lu-PSMA-617
- Underlying molecular factors that may confer radioresistance
LuCAB is recruiting 35 to 40 mCRPC patients with disease progression after docetaxel and an ARPI and who have PSMA-avid disease (SUVmax ≥15 and no FDG PET/CT discordant disease) to receive up to 6 cycles of 177Lu-PSMA-617 7.4 GBq intravenously six weekly plus cabazitaxel at escalating doses as illustrated below. The primary study objective is to establish whether 177Lu-PSMA-617 and cabazitaxel can be combined and used at standard doses. Secondary outcomes include anti-tumor activity, patient-reported outcomes, and side effects:

Conclusions
The field of radioligand therapy has experienced significant growth over the past decade. While only two agents (Radium-223 and 177Lu-PSMA-617) are currently approved for the treatment of mCRPC patients, additional options, including 225Ac, are emerging and demonstrate promising efficacy and safety outcomes in heavily pre-treated settings. Furthermore, novel radioligand therapy combinations are emerging to overcome resistance mechanisms of current radioligand therapies.
Published August 2024

