The Prostate Cancer Genetic Risk Evaluation and Screening Study (PROGRESS) aims to define the natural history of men at high genetic risk for prostate cancer and evaluate the utility of prostate MRI as a screening tool. In cohort A of the study, participants aged 35-74 years with a pathogenic germline mutation in any of 19 prostate cancer risk genes undergo screening with prostate MRI every 3 years along with an annual prostate exam (digital rectal exam [DRE]) and PSA. Patients with abnormal DRE, elevated PSA above age-adjusted thresholds, or PI-RADS ≥3 lesions on MRI are offered prostate biopsy. In our recent publication, we report results from the first 101 participants of PROGRESS who completed the initial round of screening.5 Most patients carry pathogenic mutations in BRCA1 or BRCA2 (79 of 101) followed by ATM, CHEK2 and MSH2. In the first round of screening, 24 patients had at least one abnormal screening result, with seventeen demonstrating abnormal MRI (PIRADS ≥3), and thirteen with elevated age-adjusted PSA. Among patients with abnormal measures, 21 elected to pursue biopsy, leading to nine prostate cancers diagnosed, seven of which were clinically significant.
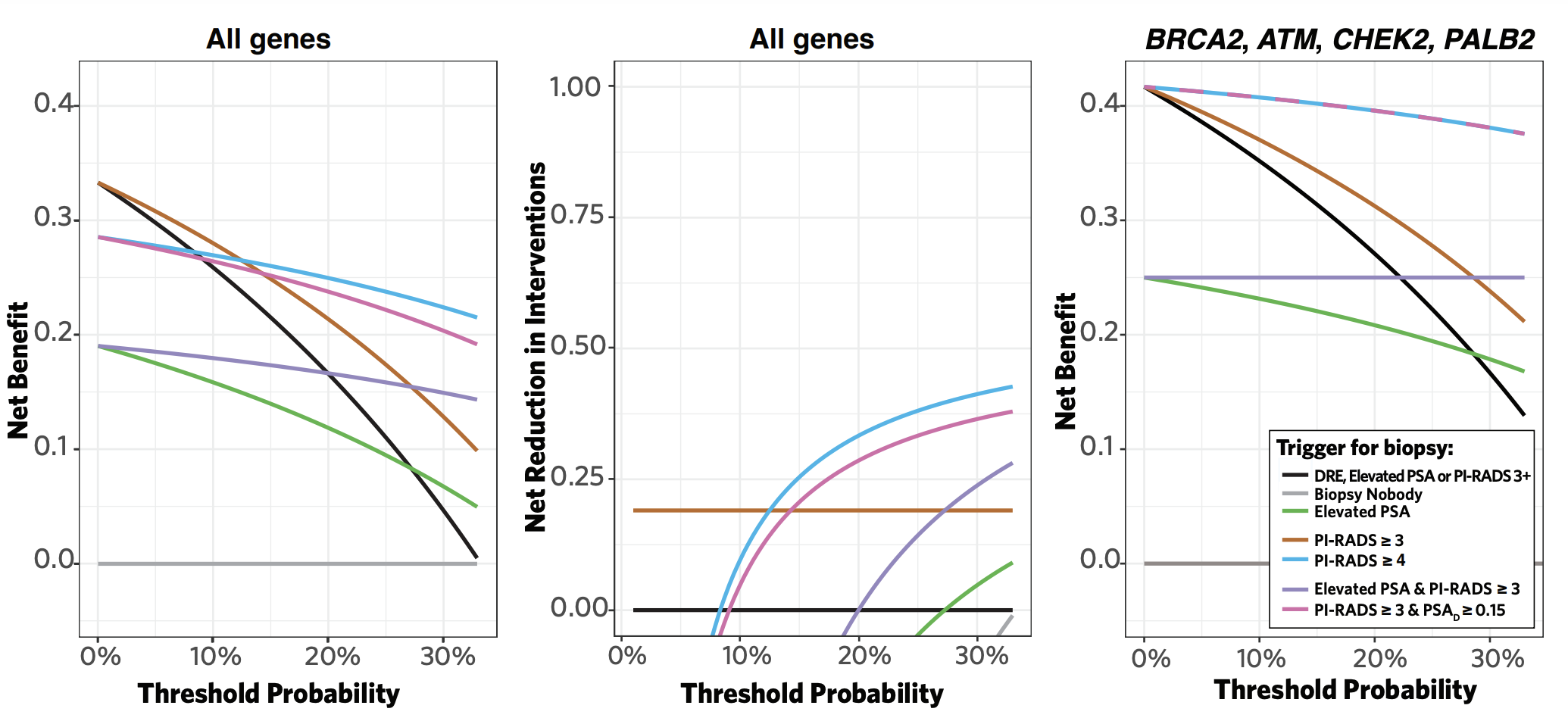
By using the prostate exam, PSA, and MRI as part of our screening protocol, we were able to compare a variety of hypothetical screening strategies (i.e., PSA only, MRI only, or combinations thereof). PSA alone detected four of the nine cancers but missed three clinically significant cancers (sensitivity, 57.1%). Meanwhile, MRI alone (PI-RADS ≥3 as a threshold for biopsy) detected all nine prostate cancers (sensitivity, 100%), and triggered a lower proportion of unnecessary biopsies than a PSA-based strategy (6/10 versus 8/17 biopsies). Decision curve analysis demonstrated MRI-based screening (with either PI-RADS ≥3 and ≥4 as a threshold for biopsy) achieved the greatest net clinical benefit compared to all other screening strategies (Figure). The net clinical benefit was even greater when restricting to patients who carry pathogenic mutations in high-evidence genes (i.e., BRCA2, CHEK2, ATM, PALB2).
Overall, we observed a high prevalence of prostate cancer (9% overall and 7% clinically significant) despite a heavily pre-screened cohort (72% of participants had PSA testing prior to enrollment). We did not diagnose any cancers among patients under 40 years old, supporting the concept of initiating screening at age 40 yr for high-risk individuals. Thus far, our interim results suggest MRI-based screening strategies enhance early detection of prostate cancer in carriers of pathogenic germline mutations. As the study continues, additional rounds of screening and longer follow-up will help refine our estimates of prostate cancer risk among mutation carriers across the heterogeneous panel of risk genes and the benefit of MRI-based screening in such high-risk populations.
Written by: Andrew E. Amini,1 & Keyan Salari,2
- Department of Urology, Massachusetts General Hospital, Harvard Medical School, Boston, MA
- Department of Urology, Massachusetts General Hospital, Harvard Medical School, Boston, MA; Center for Cancer Risk Assessment, Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, MA; Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA; Broad Institute of MIT and Harvard, Cambridge, MA
- Page EC, Bancroft EK, Brook MN, et al: Interim Results from the IMPACT Study: Evidence for Prostate-specific Antigen Screening in BRCA2 Mutation Carriers. Eur Urol 2019; 76: 831–842.
- Moses KA, Sprenkle PC, Bahler C, et al: NCCN Guidelines® Insights: Prostate Cancer Early Detection, Version 1.2023. Journal of the National Comprehensive Cancer Network 2023; 21: 236–246.
- Wei JT, Barocas D, Carlsson S, et al: Early Detection of Prostate Cancer: AUA/SUO Guideline Part I: Prostate Cancer Screening. Journal of Urology 2023; 210: 46–53.
- Mottet N, Cornford P, van den Bergh RCN, et al: EAU - EANM - ESTRO - ESUR - ISUP - SIOG Guidelines on Prostate Cancer. Milan; 2023.
- Amini AE, Hunter AE, Almashad A, et al: Magnetic Resonance Imaging–based Prostate Cancer Screening in Carriers of Pathogenic Germline Mutations: Interim Results from the Initial Screening Round of the Prostate Cancer Genetic Risk Evaluation and Screening Study. Eur Urol Oncol. 2024 Mar 6:S2588-9311(24)00041-5. doi: 10.1016/j.euo.2024.01.015. Online ahead of print.


