Advanced renal cell carcinoma (aRCC) care and treatment is constantly evolving. This was marked by the approval of multiple new agents over the last 20 years, including the approval of the vascular endothelial growth factor receptor (VEGF-R) targeting tyrosine kinase inhibitors (TKIs), as well as the introduction of mTOR inhibitors in clinical practice, followed by the approval of immunotherapy (IO), in particular checkpoint inhibitors.
The US Food and Drug Administration's approval of the combination axitinib plus pembrolizumab in 2019 marked the emergence of the use of combination IO/TKI agents in the front-line setting for the treatment of people with aRCC. , With the emergence of IO/TKI combinations comes the complexity of potential overlapping treatment-related adverse events (TRAEs). Effective management of TRAEs is needed to optimize treatment adherence and duration, and consequently improve clinical outcomes while preserving quality of life of patients receiving these efficacious treatment approaches.
Potential TRAEs
Receptor tyrosine kinases are involved in the growth and division of different cells within the body. By inhibiting VEGF-R TKIs, growth of new blood vessels slows down, thus hindering the ability of cancer cells to obtain the necessary nutrients and oxygen required to grow. The most common and clinically relevant TRAEs associated with antiangiogenic agents, such as TKIs, are diarrhea, fatigue, hypertension, nausea, and hand-foot syndrome. In contrast, TRAEs associated with IO agents are typically mediated by the immune system. Despite the differences in the mechanism of action between the two treatment classes, overlapping toxicities are often observed. Common (>10%) TRAEs that are overlapping and may be IO- or axitinib-related include hypothyroidism and hyperthyroidism, rash/inflammatory dermatitis and pruritus, diarrhea, increased alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST), nausea, arthralgia, and general disorders such as fatigue.4
This ever-evolving complexity of treatment options for patients with cancer diagnoses over the last few decades has led to the establishment of a multidisciplinary approach to patient care and management. Up to 2 of every 3 patients has an underlying comorbidity that may need to be managed in addition to their cancer diagnosis. The goal of multidisciplinary teams is to provide multiple perspective on diagnosis, aid treating physicians to remain on top of updates to clinical guidelines, and specialized treatment management based on expertise. One key individual, and often considered the glue in clinical practices serving as a messenger and important care provider to patients, is the nurse.
Role of Nurses
The role of nurses in oncology has evolved and expanded over the years. Nurse-led patient care and AE management has become a staple in current clinical practice given they are often the health care provider that spends the most time with a patient. Through collaborative efforts, oncology nurses have formulated immunotherapy management guidelines and recommendations for AEs management, as well as patient-directed materials to help improve treatment tolerability. Parreira and colleagues have expanded on these guidelines to include TKI/IO AE management recommendations.
Recommendations for Nurse Management of IO/TKI Adverse Events
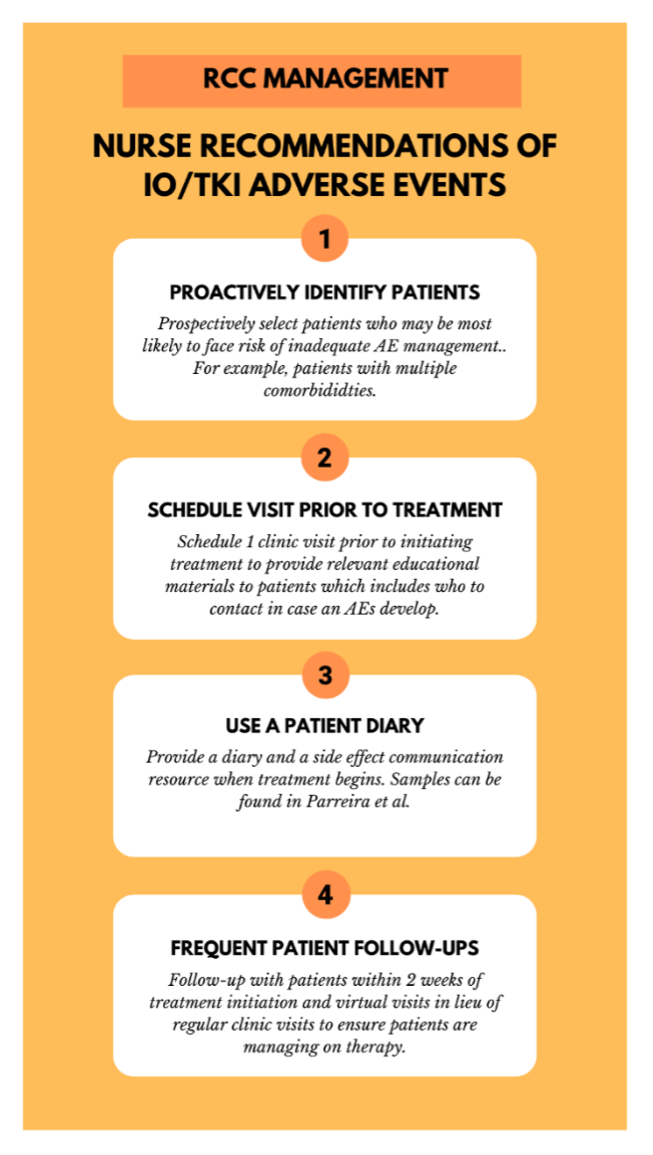
Management of Specific TRAEs
It is important to help patients identify AEs early and take appropriate measures to minimize side effects that may impact quality of life and lead to early treatment discontinuation, which could compromise their oncological outcomes. Identifying the AE is the first step in managing therapy. After the event is identified, different recommendations and guidelines for management are available, as they have been previously published.4,8
In the absence of severe symptoms that require treatment to be held, axitinib treatment interruption for 24-48 hours with patient monitoring may lead to reduced symptoms, if the TRAE is axitinib-related. A previous study demonstrated that axitinib treatment interruption of 5 of the most common AEs associated with axitinib treatment (diarrhea, fatigue, hypertension, nausea, and hand-foot syndrome) found that time to treatment resolution was generally shorter for axitinib monotherapy (≤3 days, except for fatigue) compared with axitinib + IO combinations (4-11 days).4 Parreira et al further describe nurse-assisted management of these adverse events (Figure).
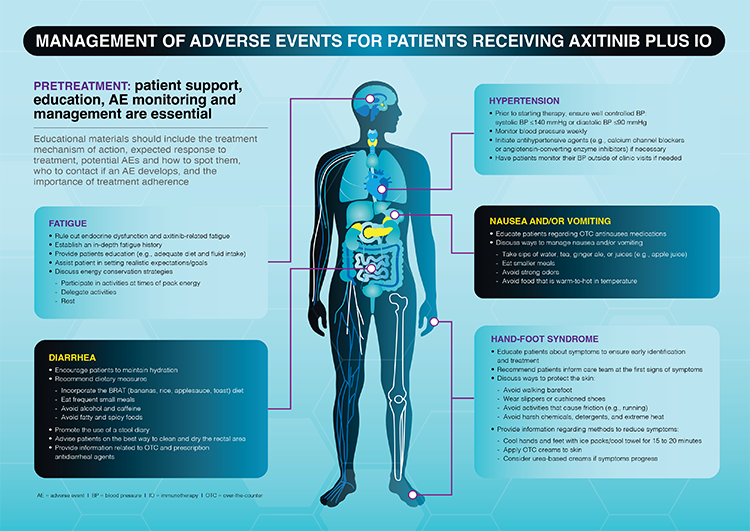
Conclusion
Ongoing education of nurses related to regular monitoring/assessment of AEs of aRCC patients receiving TKI inhibitors in combination with immunotherapy should be a priority. By prioritizing regular patient monitoring, healthcare providers can help secure efficient safety management and optimal cancer care for patients facing an aRCC diagnosis.
Written by: Laura S. Wood, RN, MSN, OCN, Cleveland Clinic Cancer Center, Cleveland, OH (retired)
References:
- Motzer RJ, Jonasch E, Agarwal N, et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2022;20(1):71-90.
- Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol 2019;30(5):706-720.
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36(17):1714-1768.
- Rini BI, Atkins MB, Choueiri TK, et al. Plain language summary looking at how long side effects last after treatment with axitinib is stopped in people with advanced renal cell carcinoma? Future Oncol. 2023 Aug 1.
- Grunwald V, Voss MH, Rini BI, et al. Axitinib plus immune checkpoint inhibitor: evidence- and expert-based consensus recommendation for treatment optimisation and management of related adverse events. Br J Cancer 2020;123(6):898-904.
- Fowler H, Belot A, Ellis L, et al. Comorbidity prevalence among cancer patients: a population-based cohort study of four cancers. 2020 Jan 28;20(1):2.
- Ornstein MC, Wood LS. Podcast to Discuss Therapy Management for Patients with Metastatic Renal Cell Carcinoma Receiving First-Line Axitinib Plus an Immune Checkpoint Inhibitor. Adv Ther. 2023 Sep;40(9):3599-3609.
- Shankar A, Wallbridge IG, Yau C, et al. Development of management strategies for immune-related adverse effects of immunotherapies used in oncological treatment. Asia Pac J Oncol Nurs 2022;9(1):7-11.
- Parreira S, Burns K, Moldawer N, et al. The role of nurses in the management of adverse events in patients receiving first-line axitinib plus immuno-oncology agents for advanced renal cell carcinoma. Semin Oncol Nurs. 2023 Nov 25:151545.
- INLYTA (axitinib) Package Insert. New York, NY: Pfizer, Inc.; 2022.
- KEYTRUDA (pembrolizumab) Package Insert. Rahway, NJ: Merck & Co., Inc.; 2023.
- BAVENCIO (avelumab) Package Insert. Rockland, MA: EMD Serono, Inc.; 2023.
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36(17):1714-1768.
- Shankar A, Wallbridge IG, Yau C, et al. Development of management strategies for immune-related adverse effects of immunotherapies used in oncological treatment. Asia Pac J Oncol Nurs 2022;9(1):7-11.
- Rini BI, Atkins MB, Choueiri TK, et al. Time to resolution of axitinib-related adverse events after treatment interruption in patients with advanced renal cell carcinoma. Clin Genitourin Cancer 2021;19(5):e306-e312.


