(UroToday.com) The 2023 ESMO annual meeting included a session on prostate cancer, featuring a discussant presentation by Dr. Boris Hadaschik. For his presentation, Dr. Hadaschik discussed three abstracts “HRQoL in nonmetastatic hormone-sensitive prostate cancer patients with high-risk BCR from the EMBARK study”
presented by Dr. Stephen Freedland, abstract “External validation of a digital pathology-based multimodal artificial intelligence (MMAI)-derived model in high-risk M0/M1 prostate cancer starting ADT in the docetaxel or abiraterone phase 3 STAMPEDE trials” presented by Dr. Charles Parker, and abstract “Incidence of fracture related hospitalizations in men with de novo high risk localized and metastatic hormone sensitive prostate cancer: Analysis of routinely collected healthcare data from the STAMPEDE docetaxel and zoledronic acid comparisons” presented by Dr. Craig Jones.
Discussing the EMBARK trial, Dr. Hadaschik notes that the EAU guidelines suggest we should perform a PSMA PET/CT if the PSA is > 0.2 ng/mL and if the results will influence treatment decisions. For patients after radical prostatectomy that have biochemical recurrence, we should offer monitoring, including PSA for EAU low-risk BCR patients, and offer early salvage radiotherapy to men with two consecutive PSA rises. Additionally, for men with BCR after radiotherapy, we should only offer salvage radical prostatectomy, brachytherapy, HIFU, or cryoablation to highly selected patients with biopsy proven local recurrence with a clinical trial setting or well-designed prospective cohort study undertaken at experienced centers. With regards to systemic salvage treatment, we should offer ADT to M0 patients with a PSA doubling time > 12 months.
In EMBARK, nonmetastatic hormone-sensitive prostate cancer patients with high-risk BCR (PSA doubling time ≤9 months, screening PSA ≥2 ng/mL above nadir post radiotherapy or ≥1 ng/mL post radical prostatectomy) were randomized (1:1:1) to enzalutamide + leuprolide acetate, enzalutamide alone, and placebo + leuprolide acetate. The trial design for EMBARK is as follows:
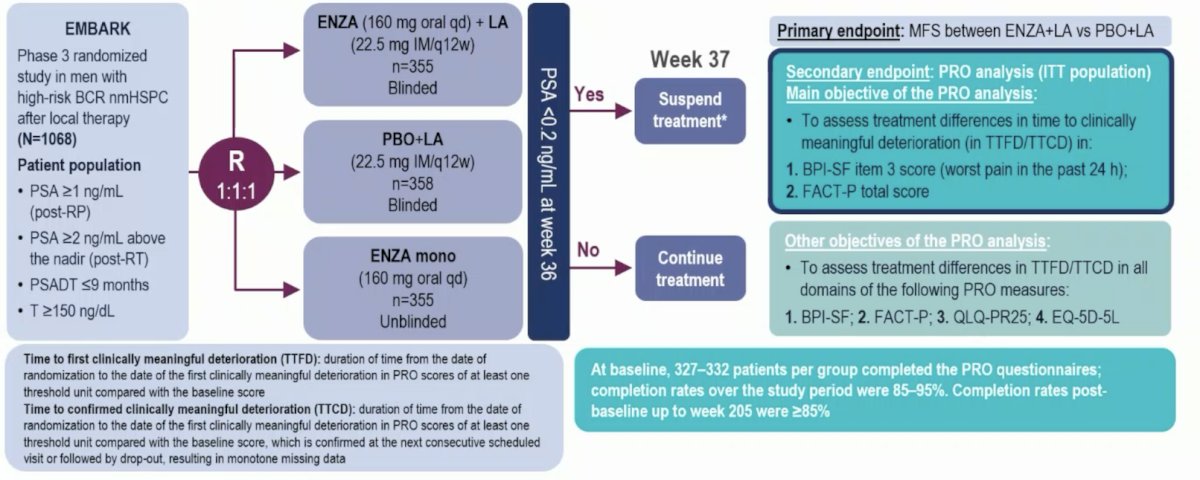
Dr. Hadaschik notes that is important that both enzalutamide + leuprolide acetate and enzalutamide alone delayed metastasis-free survival (MFS).1 With regards to treatment emergent events of special interest, for patients that do not have detectable cancer, enzalutamide is somewhat toxic with all grade cognitive and memory impairment affecting 15.0% of patients in the enzalutamide + leuprolide arm and 14.1% of patients in the enzalutamide monotherapy arm. Additionally, grade >= 3 fatigue affected 4.0% of men in the enzalutamide + leuprolide arm and 4.8% of men in the enzalutamide monotherapy arm. Because of these side effects, there was a treatment discontinuation rate of 20.7% in the enzalutamide + leuprolide arm and 17.8% in the enzalutamide monotherapy arm.
Dr. Hadaschik also emphasized several important health related quality of life outcomes from EMBARK:
- There were no significant differences in time to first deterioration or time to first clinically meaningful deterioration was seen among treatment groups vs placebo + leuprolide acetate in FACT-P total score or BPI-SF worst pain
- In the FACT-P subdomains, time to first clinically meaningful deterioration in the physical well-being subdomain score was significantly shorter for enzalutamide + leuprolide and for enzalutamide monotherapy versus placebo + leuprolide
- There was no significant difference observed in time to first deterioration or time to first clinically meaningful deterioration for other FACT-P subdomains
- Time to first clinically meaningful deterioration for hormone treatment-related symptoms was significantly shorter with enzalutamide + leuprolide acetate vs placebo + leuprolide acetate
- Time to first clinically meaningful deterioration for sexual activity was significantly longer with enzalutamide alone vs placebo + leuprolide acetate
Although the EMBARK OS data is immature at the interim analysis (48% of events), we do have data from ARCHES in the mHSPC space suggesting there is an OS benefit to treatment with enzalutamide + ADT for men with < 5 metastases.2
Dr. Hadaschik provided the following personal conclusions regarding the EMBARK trial:
- Oncological benefit and quality of life together determine clinical benefit
- Updated ESMO MCBS score cards are coming soon
- Enzalutamide + ADT improved MFS without negatively affecting overall health related quality of life (similar to M0 CRPC)
- Treatment de-escalation for good responders is highly welcome
- ADT for BCR is not standard of care – how many patients could have been treated locally after PSMA PET without or only short term systemic treatment?
- For enzalutamide monotherapy would have been nice to have less treatment emergent adverse events and quality of life benefits, but perhaps we need longer follow-up
- We need to support clinical studies with modern imaging in this disease space
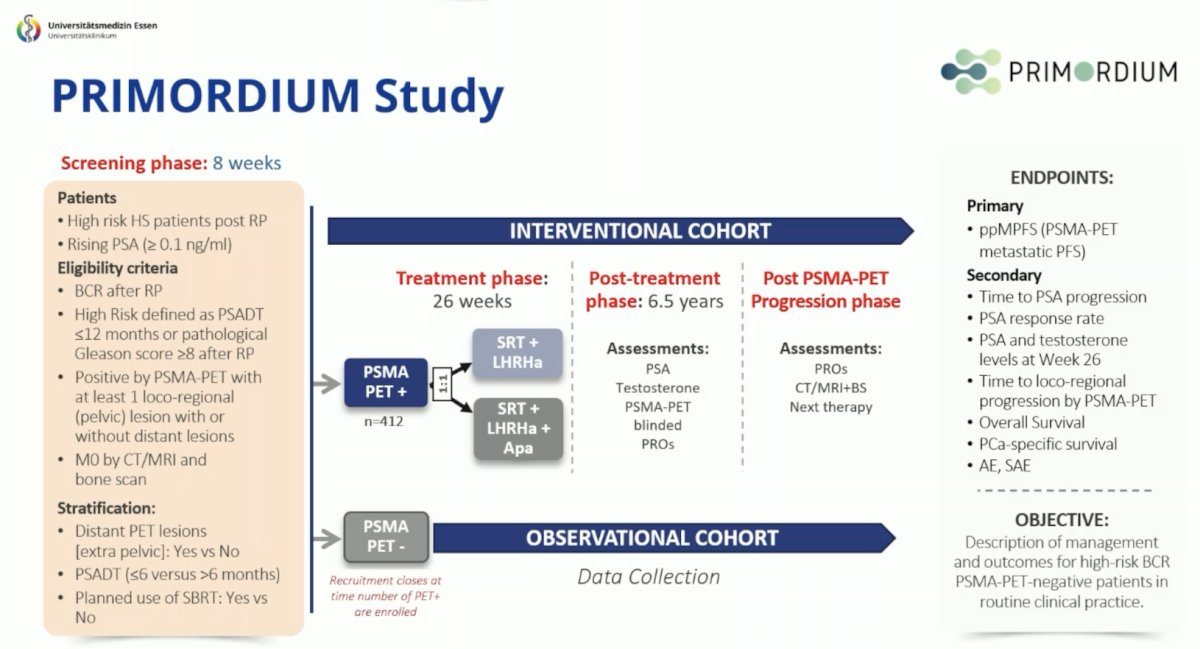
There are several clinical trials ongoing in non metastatic hormone sensitive prostate cancer, including ARASTEP with darolutamide and the PRIMORDIUM study:
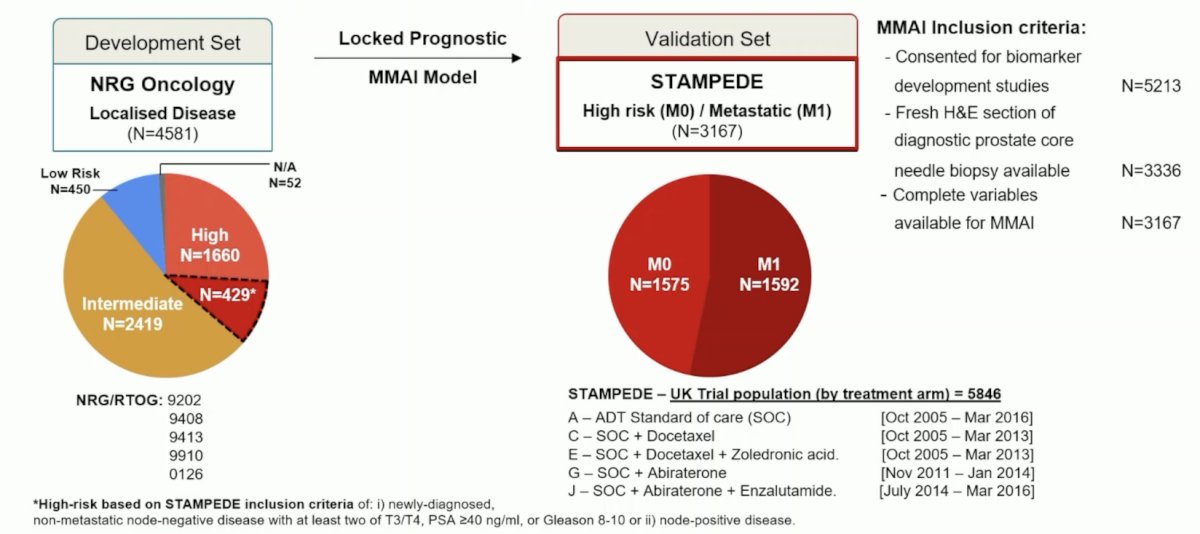
Moving to the mHSPC disease space, Dr. Parker discussed the ArteraAI validation in the STAMPEDE platform. In the last decade, we have seen a massive shift in the treatment landscape of mHSPC:

Importantly, ADT + docetaxel is no longer standard of care and the EAU guidelines state that it should only be given with abiraterone + prednisone or darolutamide based on the PEACE-13 and ARASENS4 trials, respectively. Artificial intelligence may provide an avenue for prognostication in prostate cancer and improve patient selection for those who may benefit from triplet therapy. The ArteraAI STAMPEDE study design is as follows:
Dr. Hadaschik notes that the following figures highlight the strength of the association between ArteraAI and PCSM:
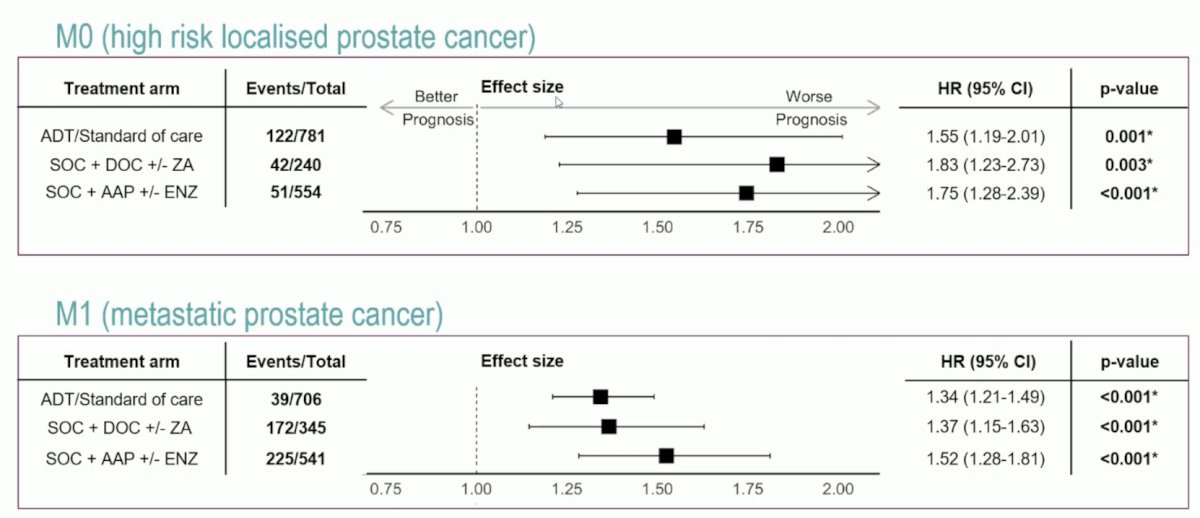
Furthermore, ArteraAI quartile 4 versus quartile 1-3 had more PCSM events at 5 years: 16% (95% CI 12-19%) versus 5% (95% CI 4-7%) in M0, 58% (95% CI 53-63%) versus 39% (95% CI 36-42%) in M1:
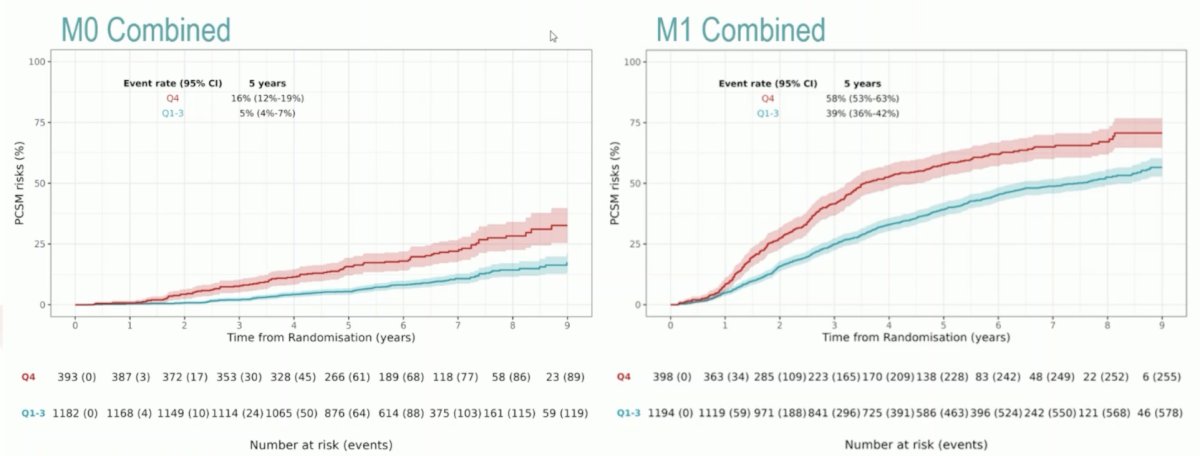
Dr. Hadaschik provided the following personal conclusions regarding the ArteraAI STAMPEDE validation study:
- Better risk stratification is very important and highly welcome to further advance the field: upfront de-escalation versus de-escalation for good responders?
- MMAI is very promising technology and the validation with STAMPEDE data is important
- MMAI allows for large scale adoption given that most slides are usable
- Applicability to MRI-targeted biopsy, prostatectomy specimens, and metastases remains to be determined
- There is still some room for improvement with the areas under the curve
- Modern imaging and genomic analyses such as Decipher may further improve prognostication
- Prospective demonstration of oncologic impact will be important
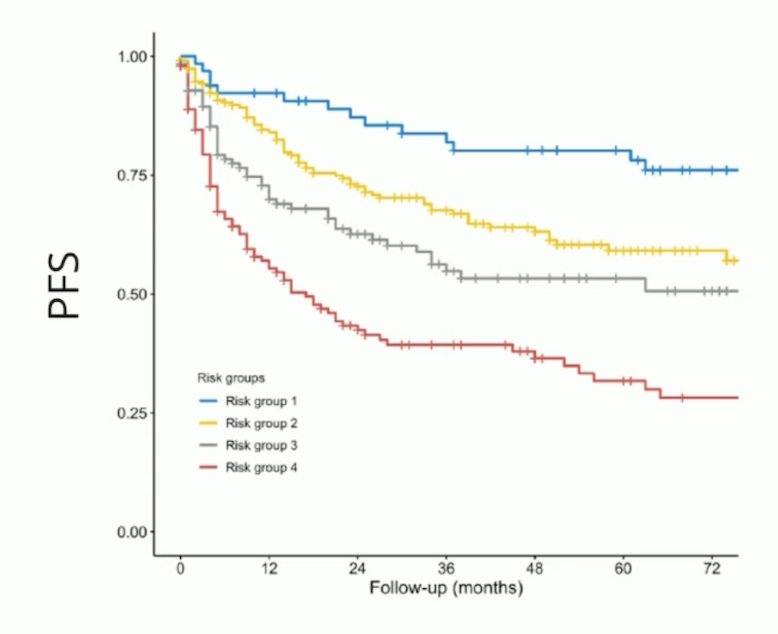
Dr. Hadaschik notes that novel EAU risk groups including MRI also provide important prognostication for local staging of prostate cancer at the time of radical prostatectomy and determining progression free survival:5
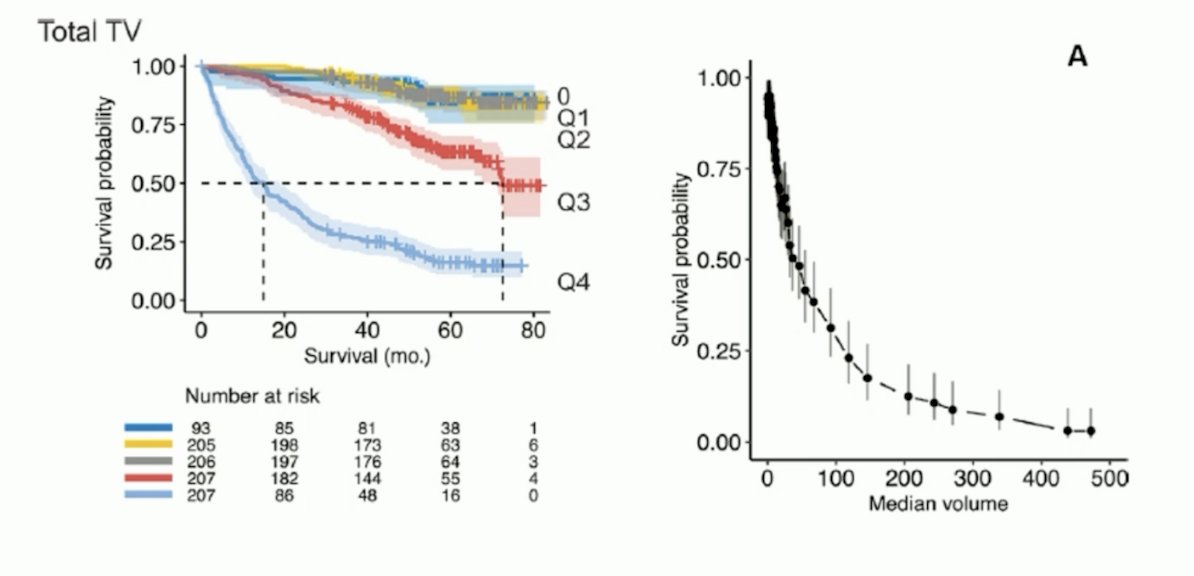
Additionally, we know that PSMA PET/CT derived tumor volume also predicts overall survival.6
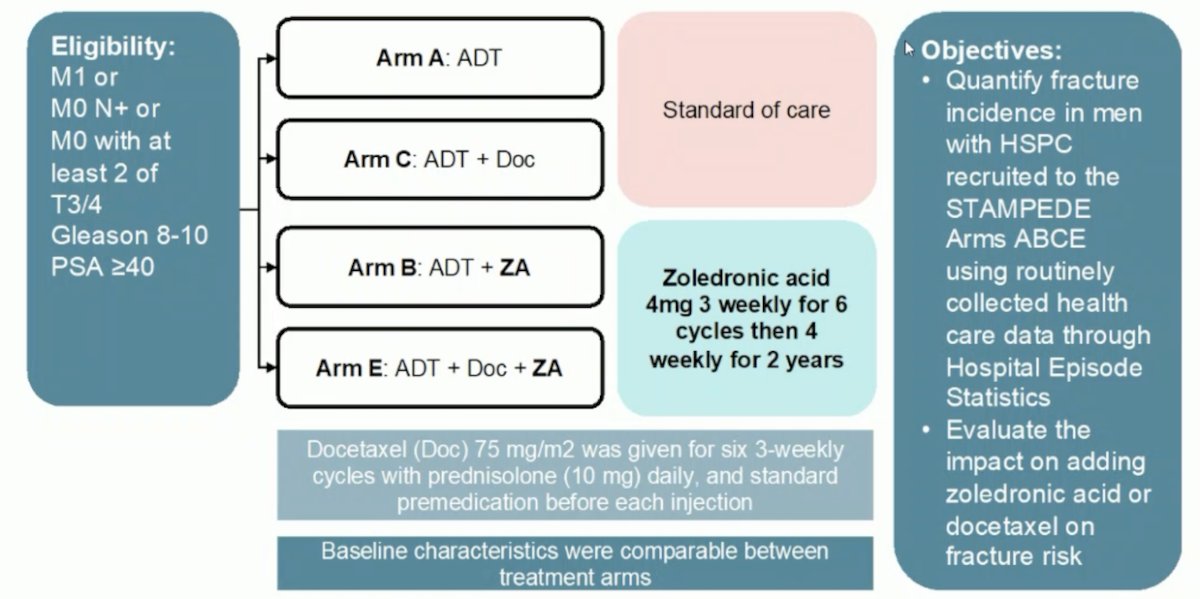
Dr. Hadaschik then discussed the risk of fracture related hospitalization of men receiving ADT + zoledronic acid and ADT + docetaxel + zoledronic acid from the STAMPEDE platform. Hospital Episode Statistics data were obtained (up to March 2021) for patients randomized to ADT (Arm A), ADT + zoledronic acid (Arm B), ADT+ docetaxel (Arm C) and ADT+ docetaxel + zoledronic acid (Arm E). Zoledronic acid (4 mg) was given in six 3 weekly cycles, then 4 weekly for 2 years:
Indeed, fractures requiring hospitalization are common: The 5-year cumulative incidence of fracture related hospitalizations for M1 and M0 patients treated with ADT were 23% (95% CI 19-28%) and 11% (95% CI 8-15%) respectively. The 10-year cumulative incidence of ADT in M0 patients was 26% (95% CI 20-33%):
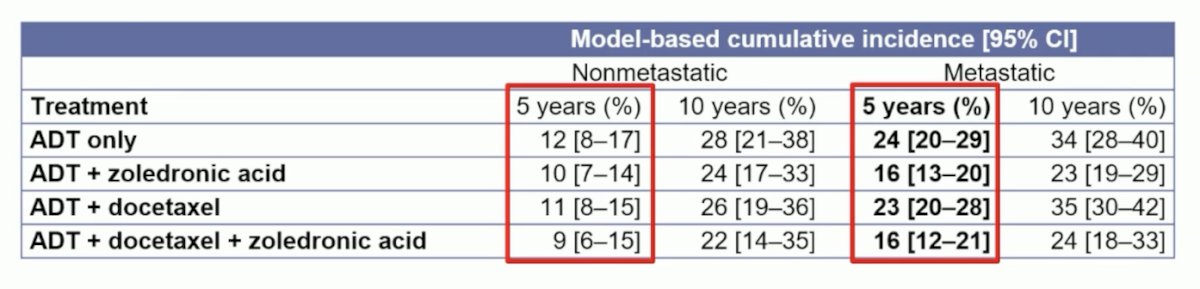
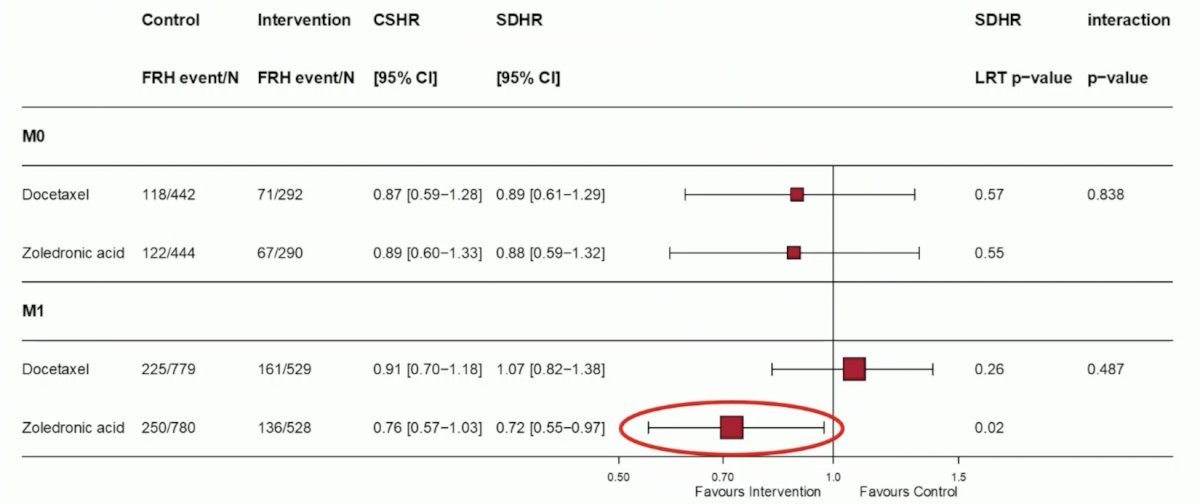
The addition of zoledronic acid significantly reduced the incidence of fracture related hospitalizations in M1 patients (sdHR 0.73, 95% CI 0.55-0.97; p=0.015), whereas data were inconclusive in M0 patients (sdHR 0.88, 95% CI 0.59-1.32, p=0.55). Docetaxel had no significant effect on fracture related hospitalizations in both M1 (p=0.264) and M0 (p=0.570) patients, with no evidence of interaction between zoledronic acid and docetaxel in both M1 (p=0.526) and M0 (p=0.805) patients:
Dr. Hadaschik concluded his discussant presentation with the following personal conclusions from the STAMPEDE fracture related hospitalization study:
- We need to pay attention to bone health and other clinically relevant consequences of treatment
- Osteoprotection in M1 disease is important, however, which drug, which schedule, and which time point are unclear
- How many patients receive osteoprotection at the time of mCRPC? This would be a great place to start
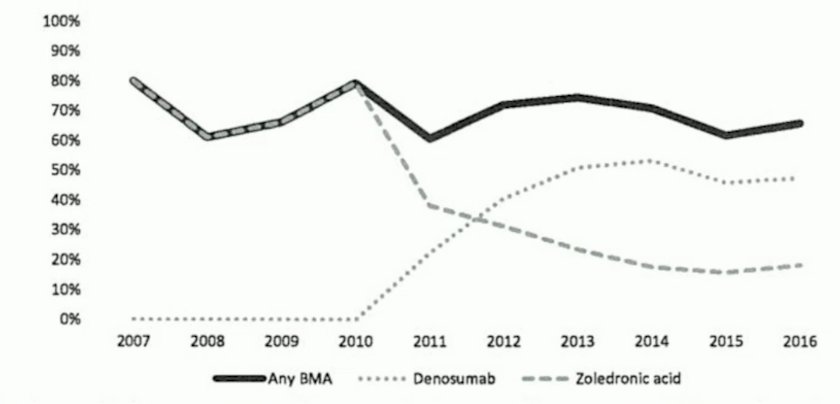
- Real world data demonstrate room for improvement:7
Presented by: Boris A. Hadaschik, MD, Essen University Hospital, Essen, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Freedland SJ, de Almeida Luz M, De Giorgi U, et al. Improved Outcomes with Enzalutamide in Biochemically Recurrent Prostate Cancer. N Engl J Med 2023 Oct 19 [Epub ahead of print].
- Armstrong AJ, Iguchi T, Azad AA, et al. The efficacy of enzalutamide plus androgen deprivation therapy in oligometastatic hormone-sensitive prostate cancer: A Post hoc analysis of ARCHES. Eur Urol. 2023 Aug;84(2):229-241.
- Fizazi K, Foulon S, Carles J, Roubaud G, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomized, phase 3 study with a 2 x 2 factorial design. Lancet. 2022 Apr 30;399(10336):1695-1707.
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142.
- Rakauskas A, Peters M, Ball D, et al. The impact of local staging of prostate cancer determined on MRI or DRE at time of radical prostatectomy on progression free survival: A Will Rogers phenomenon. Urol Oncol. 2023 Feb;41(2):106.e9-106.e16.
- Seifert R, Rasul S, Seitzer K, et al. A prognostic risk score for prostate cancer based on PSMA PET-derived organ specific tumor volumes. Radiology 2023 May;307(4):e222010.
- Mitchell AP, Meza AK, Panageas KS, Real-world use of bone modifying agents in metastatic, castration resistant prostate cancer. Prostate Cancer Prostatic Dis. 2023 Mar;26(1):126-132.
ESMO 2023: EMBARK: Health-Related Quality of Life in nmHSPC Patients with High-Risk BCR
ESMO 2023: External Validation of a Digital Pathology-Based Multimodal Artificial Intelligence (MMAI)-Derived Model in High-Risk M0/M1 Prostate Cancer Starting ADT in the Docetaxel or Abiraterone Phase 3 STAMPEDE Trials
ESMO 2023: Healthcare Data from the STAMPEDE Docetaxel and Zoledronic Acid Comparisons: Incidence of Fracture Related Hospitalisations in Men with De Novo High Risk and Metastatic Hormone Sensitive Prostate Cancer


